当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tobramycin-mediated self-assembly of DNA nanostructures for targeted treatment of Pseudomonas aeruginosa-infected lung inflammation
Biomaterials Science ( IF 6.6 ) Pub Date : 2024-03-06 , DOI: 10.1039/d3bm02121a Yuhang Xu 1 , Qian Liu 1, 2 , Bin Wang 1, 3 , Quan Li 1 , Yue Chen 1 , Yao Yang 1 , Zhihao Zhu 1 , Daohui Gong 1 , Chuan Zhang 4 , Guansong Wang 1, 3 , Hang Qian 1, 3
Biomaterials Science ( IF 6.6 ) Pub Date : 2024-03-06 , DOI: 10.1039/d3bm02121a Yuhang Xu 1 , Qian Liu 1, 2 , Bin Wang 1, 3 , Quan Li 1 , Yue Chen 1 , Yao Yang 1 , Zhihao Zhu 1 , Daohui Gong 1 , Chuan Zhang 4 , Guansong Wang 1, 3 , Hang Qian 1, 3
Affiliation
|
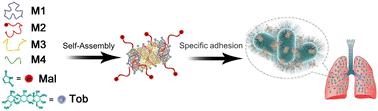
Pseudomonas aeruginosa (PA) is one of the most common multidrug-resistant pathogens found in clinics, often manifesting as biofilms. However, due to the emergence of superbugs in hospitals and the overuse of antibiotics, the prevention and treatment of PA infections have become increasingly challenging. Utilizing DNA nanostructures for packaging and delivering antibiotics presents an intervention strategy with significant potential. Nevertheless, construction of functional DNA nanostructures with multiple functionalities and enhanced stability in physiological settings remains challenging. In this study, the authors propose a magnesium-free assembly method that utilizes tobramycin (Tob) as a mediator to assemble DNA nanostructures, allowing for the functionalization of DNA nanostructures by combining DNA and antibiotics. Additionally, our study incorporates maleimide-modified DNA into the nanostructures to act as a targeting moiety specifically directed towards the pili of PA. The targeting ability of the constructed functional DNA nanostructure significantly improves the local concentration of Tob, thereby reducing the side effects of antibiotics. Our results demonstrate the successful construction of a maleimide-decorated Tob/DNA nanotube (NTTob-Mal) for the treatment of PA-infected lung inflammation. The stability and biocompatibility of NTTob-Mal are confirmed, highlighting its potential for clinical applications. Furthermore, its specificity in recognizing and adhering to PA has been validated. In vitro experiments have shown its efficacy in inhibiting PA biofilm formation, and in a murine model, NTTob-Mal has exhibited significant therapeutic effectiveness against PA-induced pneumonia. In summary, the proposed antibiotic drug-mediated DNA nanostructure assembly approach holds promise as a novel strategy for targeted treatment of PA infections.
中文翻译:

妥布霉素介导的 DNA 纳米结构自组装靶向治疗铜绿假单胞菌感染的肺部炎症
铜绿假单胞菌(PA)是临床上最常见的多重耐药病原体之一,通常表现为生物膜。然而,由于医院超级细菌的出现以及抗生素的过度使用,PA感染的预防和治疗变得越来越具有挑战性。利用 DNA 纳米结构包装和输送抗生素是一种具有巨大潜力的干预策略。然而,构建具有多种功能和增强生理环境稳定性的功能性 DNA 纳米结构仍然具有挑战性。在这项研究中,作者提出了一种无镁组装方法,利用妥布霉素 (Tob) 作为介质来组装 DNA 纳米结构,从而通过结合 DNA 和抗生素来实现 DNA 纳米结构的功能化。此外,我们的研究将马来酰亚胺修饰的 DNA 纳入纳米结构中,作为专门针对 PA 菌毛的靶向部分。所构建的功能性DNA纳米结构的靶向能力显着提高了Tob的局部浓度,从而减少了抗生素的副作用。我们的结果证明成功构建了马来酰亚胺修饰的 Tob/DNA 纳米管 (NT Tob -Mal),用于治疗 PA 感染的肺部炎症。NT Tob -Mal的稳定性和生物相容性得到证实,凸显了其临床应用潜力。此外,其识别和遵守 PA 的特异性已得到验证。体外实验已显示出其抑制 PA 生物膜形成的功效,并且在小鼠模型中,NT Tob -Mal 对 PA 诱导的肺炎表现出显着的治疗效果。总之,所提出的抗生素药物介导的 DNA 纳米结构组装方法有望成为靶向治疗 PA 感染的新策略。
更新日期:2024-03-06
中文翻译:

妥布霉素介导的 DNA 纳米结构自组装靶向治疗铜绿假单胞菌感染的肺部炎症
铜绿假单胞菌(PA)是临床上最常见的多重耐药病原体之一,通常表现为生物膜。然而,由于医院超级细菌的出现以及抗生素的过度使用,PA感染的预防和治疗变得越来越具有挑战性。利用 DNA 纳米结构包装和输送抗生素是一种具有巨大潜力的干预策略。然而,构建具有多种功能和增强生理环境稳定性的功能性 DNA 纳米结构仍然具有挑战性。在这项研究中,作者提出了一种无镁组装方法,利用妥布霉素 (Tob) 作为介质来组装 DNA 纳米结构,从而通过结合 DNA 和抗生素来实现 DNA 纳米结构的功能化。此外,我们的研究将马来酰亚胺修饰的 DNA 纳入纳米结构中,作为专门针对 PA 菌毛的靶向部分。所构建的功能性DNA纳米结构的靶向能力显着提高了Tob的局部浓度,从而减少了抗生素的副作用。我们的结果证明成功构建了马来酰亚胺修饰的 Tob/DNA 纳米管 (NT Tob -Mal),用于治疗 PA 感染的肺部炎症。NT Tob -Mal的稳定性和生物相容性得到证实,凸显了其临床应用潜力。此外,其识别和遵守 PA 的特异性已得到验证。体外实验已显示出其抑制 PA 生物膜形成的功效,并且在小鼠模型中,NT Tob -Mal 对 PA 诱导的肺炎表现出显着的治疗效果。总之,所提出的抗生素药物介导的 DNA 纳米结构组装方法有望成为靶向治疗 PA 感染的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号