当前位置:
X-MOL 学术
›
Acta Pharm. Sin. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Branched glycopolymer prodrug-derived nanoassembly combined with a STING agonist activates an immuno-supportive status to boost anti-PD-L1 antibody therapy
Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2024-03-02 , DOI: 10.1016/j.apsb.2024.02.006 Zhilin Li , Qianfeng Zhang , Zhiqian Li , Long Ren , Dayi Pan , Qiyong Gong , Zhongwei Gu , Hao Cai , Kui Luo
Acta Pharmaceutica Sinica B ( IF 14.5 ) Pub Date : 2024-03-02 , DOI: 10.1016/j.apsb.2024.02.006 Zhilin Li , Qianfeng Zhang , Zhiqian Li , Long Ren , Dayi Pan , Qiyong Gong , Zhongwei Gu , Hao Cai , Kui Luo
|
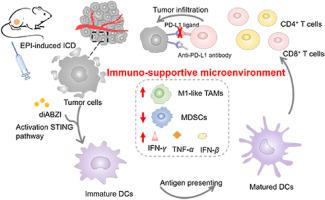
Despite the great potential of anti-PD-L1 antibodies for immunotherapy, their low response rate due to an immunosuppressive tumor microenvironment has hampered their application. To address this issue, we constructed a cell membrane-coated nanosystem (mB4S) to reverse an immunosuppressive microenvironment to an immuno-supportive one for strengthening the anti-tumor effect. In this system, Epirubicin (EPI) as an immunogenic cell death (ICD) inducer was coupled to a branched glycopolymer hydrazone bonds and diABZI as a stimulator of interferon genes (STING) agonist was encapsulated into mB4S. After internalization of mB4S, EPI was acidic-responsively released to induce ICD, which was characterized by an increased level of calreticulin (CRT) exposure and enhanced ATP secretion. Meanwhile, diABZI effectively activated the STING pathway. Treatment with mB4S in combination with an anti-PD-L1 antibody elicited potent immune responses by increasing the ratio of matured dendritic cells (DCs) and CD8 T cells, promoting cytokines secretion, up-regulating M1-like tumor-associated macrophages (TAMs) and down-regulating immunosuppressive myeloid-derived suppressor cells (MDSCs). Therefore, this nanosystem for co-delivery of an ICD inducer and a STING agonist achieved promotion of DCs maturation and CD8 T cells infiltration, creating an immuno-supportive microenvironment, thus potentiating the therapy effect of the anti-PD-L1 antibody in both 4T1 breast and CT26 colon tumor mice.
中文翻译:

分支糖聚合物前药衍生的纳米组装体与 STING 激动剂相结合可激活免疫支持状态以增强抗 PD-L1 抗体治疗
尽管抗PD-L1抗体在免疫治疗方面具有巨大潜力,但由于免疫抑制性肿瘤微环境而导致其低反应率阻碍了其应用。为了解决这个问题,我们构建了一种细胞膜涂层纳米系统(mB4S),将免疫抑制微环境逆转为免疫支持微环境,从而增强抗肿瘤效果。在该系统中,表柔比星 (EPI) 作为免疫原性细胞死亡 (ICD) 诱导剂与支链糖聚合物腙键偶联,而 diABZI 作为干扰素基因刺激剂 (STING) 激动剂被封装到 mB4S 中。 mB4S 内化后,EPI 酸性反应性释放以诱导 ICD,其特点是钙网蛋白 (CRT) 暴露水平增加和 ATP 分泌增强。同时,diABZI 有效激活了 STING 通路。 mB4S 与抗 PD-L1 抗体联合治疗可通过增加成熟树突状细胞 (DC) 和 CD8 T 细胞的比例、促进细胞因子分泌、上调 M1 样肿瘤相关巨噬细胞 (TAM) 来引发有效的免疫反应以及下调免疫抑制性骨髓源性抑制细胞(MDSC)。因此,这种共同递送 ICD 诱导剂和 STING 激动剂的纳米系统实现了促进 DC 成熟和 CD8 T 细胞浸润,创造了免疫支持微环境,从而增强了抗 PD-L1 抗体在 4T1 中的治疗效果。乳腺癌和 CT26 结肠肿瘤小鼠。
更新日期:2024-03-02
中文翻译:

分支糖聚合物前药衍生的纳米组装体与 STING 激动剂相结合可激活免疫支持状态以增强抗 PD-L1 抗体治疗
尽管抗PD-L1抗体在免疫治疗方面具有巨大潜力,但由于免疫抑制性肿瘤微环境而导致其低反应率阻碍了其应用。为了解决这个问题,我们构建了一种细胞膜涂层纳米系统(mB4S),将免疫抑制微环境逆转为免疫支持微环境,从而增强抗肿瘤效果。在该系统中,表柔比星 (EPI) 作为免疫原性细胞死亡 (ICD) 诱导剂与支链糖聚合物腙键偶联,而 diABZI 作为干扰素基因刺激剂 (STING) 激动剂被封装到 mB4S 中。 mB4S 内化后,EPI 酸性反应性释放以诱导 ICD,其特点是钙网蛋白 (CRT) 暴露水平增加和 ATP 分泌增强。同时,diABZI 有效激活了 STING 通路。 mB4S 与抗 PD-L1 抗体联合治疗可通过增加成熟树突状细胞 (DC) 和 CD8 T 细胞的比例、促进细胞因子分泌、上调 M1 样肿瘤相关巨噬细胞 (TAM) 来引发有效的免疫反应以及下调免疫抑制性骨髓源性抑制细胞(MDSC)。因此,这种共同递送 ICD 诱导剂和 STING 激动剂的纳米系统实现了促进 DC 成熟和 CD8 T 细胞浸润,创造了免疫支持微环境,从而增强了抗 PD-L1 抗体在 4T1 中的治疗效果。乳腺癌和 CT26 结肠肿瘤小鼠。



























 京公网安备 11010802027423号
京公网安备 11010802027423号