当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of Red-Shifting Mutations in Firefly Luciferase Using High-Throughput Biochemistry
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-04 , DOI: 10.1021/acs.biochem.3c00708 Clair M. Colee 1 , Nicole M. Oberlag 1 , Marcell Simon 1 , Owen S. Chapman 2 , Lyndsey C. Flanagan 1 , Edison S. Reid-McLaughlin 1 , Jordan A. Gewing-Mullins 1 , Synaida Maiche 1 , Devi F. Patel 1 , Andre R. O. Cavalcanti 2 , Aaron M. Leconte 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-04 , DOI: 10.1021/acs.biochem.3c00708 Clair M. Colee 1 , Nicole M. Oberlag 1 , Marcell Simon 1 , Owen S. Chapman 2 , Lyndsey C. Flanagan 1 , Edison S. Reid-McLaughlin 1 , Jordan A. Gewing-Mullins 1 , Synaida Maiche 1 , Devi F. Patel 1 , Andre R. O. Cavalcanti 2 , Aaron M. Leconte 1
Affiliation
|
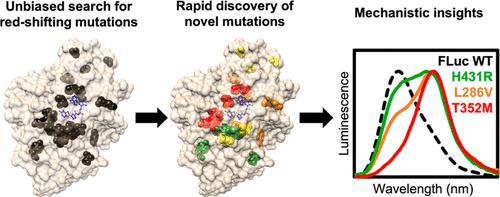
Photinus pyralis luciferase (FLuc) has proven a valuable tool for bioluminescence imaging, but much of the light emitted from the native enzyme is absorbed by endogenous biomolecules. Thus, luciferases displaying red-shifted emission enable higher resolution during deep-tissue imaging. A robust model of how protein structure determines emission color would greatly aid the engineering of red-shifted mutants, but no consensus has been reached to date. In this work, we applied deep mutational scanning to systematically assess 20 functionally important amino acid positions on FLuc for red-shifting mutations, predicting that an unbiased approach would enable novel contributions to this debate. We report dozens of red-shifting mutations as a result, a large majority of which have not been previously identified. Further characterization revealed that mutations N229T and T352M, in particular, bring about unimodal emission with the majority of photons being >600 nm. The red-shifting mutations identified by this high-throughput approach provide strong biochemical evidence for the multiple-emitter mechanism of color determination and point to the importance of a water network in the enzyme binding pocket for altering the emitter ratio. This work provides a broadly applicable mutational data set tying FLuc structure to emission color that contributes to our mechanistic understanding of emission color determination and should facilitate further engineering of improved probes for deep-tissue imaging.
中文翻译:

利用高通量生物化学发现萤火虫荧光素酶红移突变
萤火虫萤光素酶 (FLuc) 已被证明是生物发光成像的宝贵工具,但天然酶发出的大部分光被内源生物分子吸收。因此,显示红移发射的荧光素酶能够在深部组织成像过程中实现更高的分辨率。蛋白质结构如何决定发射颜色的强大模型将极大地有助于红移突变体的工程设计,但迄今为止尚未达成共识。在这项工作中,我们应用深度突变扫描来系统地评估 FLuc 上 20 个功能重要的氨基酸位置的红移突变,预测公正的方法将为这场争论做出新的贡献。结果,我们报告了数十种红移突变,其中大部分以前未被识别。进一步的表征表明,突变 N229T 和 T352M 尤其会导致单峰发射,大多数光子> 600 nm。通过这种高通量方法鉴定的红移突变为颜色测定的多发射体机制提供了强有力的生化证据,并指出了酶结合口袋中的水网络对于改变发射体比率的重要性。这项工作提供了一个广泛适用的突变数据集,将 FLuc 结构与发射颜色联系起来,有助于我们对发射颜色测定的机制理解,并应有助于进一步设计用于深层组织成像的改进探针。
更新日期:2024-03-04
中文翻译:

利用高通量生物化学发现萤火虫荧光素酶红移突变
萤火虫萤光素酶 (FLuc) 已被证明是生物发光成像的宝贵工具,但天然酶发出的大部分光被内源生物分子吸收。因此,显示红移发射的荧光素酶能够在深部组织成像过程中实现更高的分辨率。蛋白质结构如何决定发射颜色的强大模型将极大地有助于红移突变体的工程设计,但迄今为止尚未达成共识。在这项工作中,我们应用深度突变扫描来系统地评估 FLuc 上 20 个功能重要的氨基酸位置的红移突变,预测公正的方法将为这场争论做出新的贡献。结果,我们报告了数十种红移突变,其中大部分以前未被识别。进一步的表征表明,突变 N229T 和 T352M 尤其会导致单峰发射,大多数光子> 600 nm。通过这种高通量方法鉴定的红移突变为颜色测定的多发射体机制提供了强有力的生化证据,并指出了酶结合口袋中的水网络对于改变发射体比率的重要性。这项工作提供了一个广泛适用的突变数据集,将 FLuc 结构与发射颜色联系起来,有助于我们对发射颜色测定的机制理解,并应有助于进一步设计用于深层组织成像的改进探针。



























 京公网安备 11010802027423号
京公网安备 11010802027423号