当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Self-assembly of amphiphilic helical-coiled peptide nanofibers and inhibition of fibril formation with curcumin
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-03-01 , DOI: 10.1016/j.bmcl.2024.129682 Grace Daniel , George Hilan , Lisa Ploeg , David Sabatino
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-03-01 , DOI: 10.1016/j.bmcl.2024.129682 Grace Daniel , George Hilan , Lisa Ploeg , David Sabatino
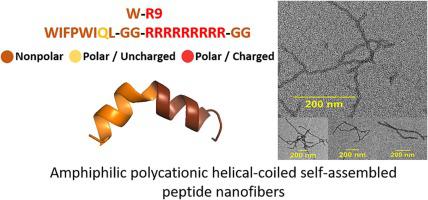
|
Amphiphilic peptide sequences are conducive to secondary structures that self-assemble into higher-ordered peptide nanostructures. A select set of amphiphilic polycationic peptides displayed stable helical-coiled structures that self-assembled into peptide nanofibers. The progression of peptide fibril formation revealed short protofibrils that extended into thin filaments and into an entangled network of nanofibers over an extended (5 days) incubation period. Ligand binding with 8-anilinonaphthalene-1-sulfonic acid (ANS) and Congo Red (CR) confirmed the amphiphilic helical-coiled peptide structure assembly into nanofibers, whereas curcumin treatment led to inhibition of fibril formation. Considering the vast repertoire of fibrous biomaterials and peptide or protein (mis)folding contingent on fibril formation, this work relates the molecular interplay in between sequence composition, structural folding and the ligand binding events impacting peptide self-assembly into nanofibers.
中文翻译:

两亲性螺旋卷曲肽纳米纤维的自组装及姜黄素对原纤维形成的抑制
两亲性肽序列有利于二级结构自组装成更高级的肽纳米结构。一组选定的两亲性聚阳离子肽表现出稳定的螺旋卷曲结构,可自组装成肽纳米纤维。肽原纤维形成的过程揭示了短的原原纤维,在延长的(5天)潜伏期内延伸成细丝并形成纳米纤维的缠结网络。配体与 8-苯胺萘-1-磺酸 (ANS) 和刚果红 (CR) 的结合证实了两亲性螺旋卷曲肽结构组装成纳米纤维,而姜黄素处理导致原纤维形成的抑制。考虑到纤维生物材料和肽或蛋白质(错误)折叠对原纤维形成的影响,这项工作涉及序列组成、结构折叠和影响肽自组装成纳米纤维的配体结合事件之间的分子相互作用。
更新日期:2024-03-01
中文翻译:

两亲性螺旋卷曲肽纳米纤维的自组装及姜黄素对原纤维形成的抑制
两亲性肽序列有利于二级结构自组装成更高级的肽纳米结构。一组选定的两亲性聚阳离子肽表现出稳定的螺旋卷曲结构,可自组装成肽纳米纤维。肽原纤维形成的过程揭示了短的原原纤维,在延长的(5天)潜伏期内延伸成细丝并形成纳米纤维的缠结网络。配体与 8-苯胺萘-1-磺酸 (ANS) 和刚果红 (CR) 的结合证实了两亲性螺旋卷曲肽结构组装成纳米纤维,而姜黄素处理导致原纤维形成的抑制。考虑到纤维生物材料和肽或蛋白质(错误)折叠对原纤维形成的影响,这项工作涉及序列组成、结构折叠和影响肽自组装成纳米纤维的配体结合事件之间的分子相互作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号