当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Identification of two novel chemical classes of Autotaxin (ATX) inhibitors using Enalos Asclepios KNIME nodes
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-03-05 , DOI: 10.1016/j.bmcl.2024.129690 Elli-Anna Stylianaki , Varnavas D. Mouchlis , Christiana Magkrioti , Konstantinos D. Papavasileiou , Antreas Afantitis , Alexios N. Matralis , Vassilis Aidinis
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-03-05 , DOI: 10.1016/j.bmcl.2024.129690 Elli-Anna Stylianaki , Varnavas D. Mouchlis , Christiana Magkrioti , Konstantinos D. Papavasileiou , Antreas Afantitis , Alexios N. Matralis , Vassilis Aidinis
|
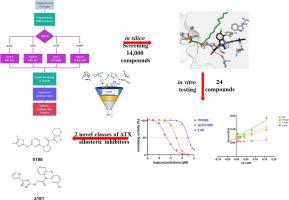
Autotaxin is a secreted lysophospholipase D which is a member of the ectonucleotide pyrophosphatase/phosphodiesterase family converting extracellular lysophosphatidylcholine and other non-choline lysophospholipids, such as lysophosphatidylethanolamine and lysophosphatidylserine, to the lipid mediator lysophosphatidic acid. Autotaxin is implicated in various fibroproliferative diseases including interstitial lung diseases, such as idiopathic pulmonary fibrosis and hepatic fibrosis, as well as in cancer. In this study, we present an effort of identifying ATX inhibitors that bind to allosteric ATX binding sites using the Enalos Asclepios KNIME Node. All the available PDB crystal structures of ATX were collected, prepared, and aligned. Visual examination of these structures led to the identification of four crystal structures of human ATX co-crystallized with four known inhibitors. These inhibitors bind to five binding sites with five different binding modes. These five binding sites were thereafter used to virtually screen a compound library of 14,000 compounds to identify molecules that bind to allosteric sites. Based on the binding mode and interactions, the docking score, and the frequency that a compound comes up as a top-ranked among the five binding sites, 24 compounds were selected for testing. Finally, two compounds emerged with inhibitory activity against ATX in the low micromolar range, while their mode of inhibition and binding pattern were also studied. The two derivatives identified herein can serve as “hits” towards developing novel classes of ATX allosteric inhibitors.
中文翻译:

使用 Enalos Asclepios KNIME 节点鉴定两种新型化学类别的自分泌运动因子 (ATX) 抑制剂
自分泌运动因子是一种分泌型溶血磷脂酶 D,它是外核苷酸焦磷酸酶/磷酸二酯酶家族的成员,可将细胞外溶血磷脂酰胆碱和其他非胆碱溶血磷脂(例如溶血磷脂酰乙醇胺和溶血磷脂酰丝氨酸)转化为脂质介质溶血磷脂酸。自分泌运动因子与多种纤维增殖性疾病有关,包括间质性肺病,如特发性肺纤维化和肝纤维化,以及癌症。在这项研究中,我们尝试使用 Enalos Asclepios KNIME Node 来鉴定与变构 ATX 结合位点结合的 ATX 抑制剂。收集、制备和排列所有可用的 ATX PDB 晶体结构。通过对这些结构的目视检查,鉴定出了与四种已知抑制剂共结晶的人 ATX 的四种晶体结构。这些抑制剂以五种不同的结合模式结合到五个结合位点。此后,这五个结合位点被用来虚拟筛选包含 14,000 种化合物的化合物库,以识别与变构位点结合的分子。根据结合模式和相互作用、对接分数以及化合物在五个结合位点中排名最高的频率,选择了 24 种化合物进行测试。最后,出现了两种在低微摩尔范围内对ATX具有抑制活性的化合物,同时还研究了它们的抑制模式和结合模式。本文确定的两种衍生物可以作为开发新型 ATX 变构抑制剂的“热门产品”。
更新日期:2024-03-05
中文翻译:

使用 Enalos Asclepios KNIME 节点鉴定两种新型化学类别的自分泌运动因子 (ATX) 抑制剂
自分泌运动因子是一种分泌型溶血磷脂酶 D,它是外核苷酸焦磷酸酶/磷酸二酯酶家族的成员,可将细胞外溶血磷脂酰胆碱和其他非胆碱溶血磷脂(例如溶血磷脂酰乙醇胺和溶血磷脂酰丝氨酸)转化为脂质介质溶血磷脂酸。自分泌运动因子与多种纤维增殖性疾病有关,包括间质性肺病,如特发性肺纤维化和肝纤维化,以及癌症。在这项研究中,我们尝试使用 Enalos Asclepios KNIME Node 来鉴定与变构 ATX 结合位点结合的 ATX 抑制剂。收集、制备和排列所有可用的 ATX PDB 晶体结构。通过对这些结构的目视检查,鉴定出了与四种已知抑制剂共结晶的人 ATX 的四种晶体结构。这些抑制剂以五种不同的结合模式结合到五个结合位点。此后,这五个结合位点被用来虚拟筛选包含 14,000 种化合物的化合物库,以识别与变构位点结合的分子。根据结合模式和相互作用、对接分数以及化合物在五个结合位点中排名最高的频率,选择了 24 种化合物进行测试。最后,出现了两种在低微摩尔范围内对ATX具有抑制活性的化合物,同时还研究了它们的抑制模式和结合模式。本文确定的两种衍生物可以作为开发新型 ATX 变构抑制剂的“热门产品”。



























 京公网安备 11010802027423号
京公网安备 11010802027423号