当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis, biological evaluation and docking study of some new aryl and heteroaryl thiomannosides as FimH antagonists
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-02 , DOI: 10.1016/j.bioorg.2024.107258 Anber F. Mohammed , Shimaa A. Othman , Ola F. Abou-Ghadir , Ahmed A. Kotb , Yaser A. Mostafa , Mohamed A. El-Mokhtar , Hajjaj H.M. Abdu-Allah
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-02 , DOI: 10.1016/j.bioorg.2024.107258 Anber F. Mohammed , Shimaa A. Othman , Ola F. Abou-Ghadir , Ahmed A. Kotb , Yaser A. Mostafa , Mohamed A. El-Mokhtar , Hajjaj H.M. Abdu-Allah
|
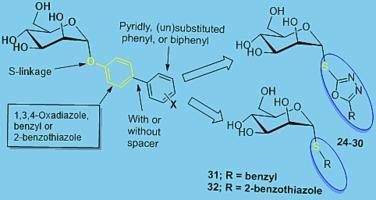
FimH is a mannose-recognizing lectin that is expressed by guiding its ability to adhere and infect cells. It is involved in pathogenesis of urinary tract infections and Chron’s disease. Several X-ray structure-guided ligand design studies were extensively utilized in the discovery and optimization of small molecule aryl mannoside FimH antagonists. These antagonists retain key specific interactions of the mannose scaffolds with the FimH carbohydrate recognition domains. Thiomannosides are attractive and stable scaffolds, and this work reports the synthesis of some of their new aryl and heteroaryl derivatives as FimH antagonists. FimH-competitive binding assays as well as biofilm inhibition of the new compounds (– were determined in comparison with the reference -heptyl α--mannopyranoside (). The affinity among these compounds was found to be governed by the structure of the aryl and heteroarylf aglycones. Two compounds and revealed higher activity than . Molecular docking and total hydrophobic to topological polar surface area ratio calculations attributed to explain the obtained biological results. Finally, the SAR study suggested that introducing an aryl or heteroaryl aglycone of sufficient hydrophobicity and of proper orientation within the tyrosine binding site considerably enhance binding affinity. The potent and synthetically feasible FimH antagonists described herein hold potential as leads for the development of sensors for detection of and treatment of its diseases.
中文翻译:

一些新型芳基和杂芳基硫代甘露糖苷FimH拮抗剂的设计、合成、生物学评价和对接研究
FimH 是一种识别甘露糖的凝集素,通过引导其粘附和感染细胞的能力来表达。它涉及尿路感染和慢性病的发病机制。多项 X 射线结构引导配体设计研究被广泛用于小分子芳基甘露糖苷 FimH 拮抗剂的发现和优化。这些拮抗剂保留了甘露糖支架与 FimH 碳水化合物识别结构域的关键特异性相互作用。硫甘露糖苷是有吸引力且稳定的支架,这项工作报告了一些新的芳基和杂芳基衍生物作为 FimH 拮抗剂的合成。 FimH 竞争性结合测定以及新化合物的生物膜抑制(– 与参考 -庚基 α--吡喃甘露糖苷 () 进行比较而确定。发现这些化合物之间的亲和力受芳基和杂芳基的结构控制糖苷配基。两种化合物并显示出比 更高的活性。分子对接和总疏水性与拓扑极性表面积之比计算归因于解释所获得的生物学结果。最后,SAR 研究表明引入具有足够疏水性和正确方向的芳基或杂芳基糖苷配基本文描述的有效且合成上可行的FimH拮抗剂具有作为开发用于检测和治疗其疾病的传感器的先导的潜力。
更新日期:2024-03-02
中文翻译:

一些新型芳基和杂芳基硫代甘露糖苷FimH拮抗剂的设计、合成、生物学评价和对接研究
FimH 是一种识别甘露糖的凝集素,通过引导其粘附和感染细胞的能力来表达。它涉及尿路感染和慢性病的发病机制。多项 X 射线结构引导配体设计研究被广泛用于小分子芳基甘露糖苷 FimH 拮抗剂的发现和优化。这些拮抗剂保留了甘露糖支架与 FimH 碳水化合物识别结构域的关键特异性相互作用。硫甘露糖苷是有吸引力且稳定的支架,这项工作报告了一些新的芳基和杂芳基衍生物作为 FimH 拮抗剂的合成。 FimH 竞争性结合测定以及新化合物的生物膜抑制(– 与参考 -庚基 α--吡喃甘露糖苷 () 进行比较而确定。发现这些化合物之间的亲和力受芳基和杂芳基的结构控制糖苷配基。两种化合物并显示出比 更高的活性。分子对接和总疏水性与拓扑极性表面积之比计算归因于解释所获得的生物学结果。最后,SAR 研究表明引入具有足够疏水性和正确方向的芳基或杂芳基糖苷配基本文描述的有效且合成上可行的FimH拮抗剂具有作为开发用于检测和治疗其疾病的传感器的先导的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号