当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding and Activation of LRP1-Dependent Cell Signaling in Schwann Cells Using a Peptide Derived from the Hemopexin Domain of MMP-9
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-07 , DOI: 10.1021/acs.biochem.3c00705 John H. Kim 1 , Aashish Shivkumar 1 , Masaki Norimoto 2 , Sascha Castro Lingl 1 , Christian Seitz 1 , Rommie E. Amaro 1 , Steve L. Gonias 3 , Jerry Yang 1 , Wendy M. Campana 2, 4
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-07 , DOI: 10.1021/acs.biochem.3c00705 John H. Kim 1 , Aashish Shivkumar 1 , Masaki Norimoto 2 , Sascha Castro Lingl 1 , Christian Seitz 1 , Rommie E. Amaro 1 , Steve L. Gonias 3 , Jerry Yang 1 , Wendy M. Campana 2, 4
Affiliation
|
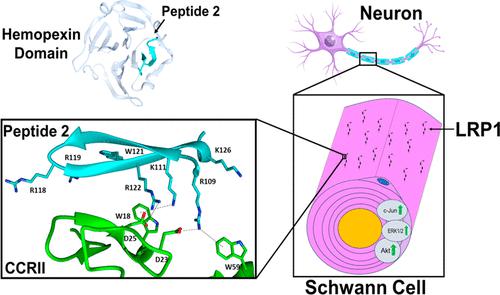
Schwann cells (SCs) undergo phenotypic transformation and then orchestrate nerve repair following a peripheral nervous system injury. The low-density lipoprotein receptor-related protein-1 (LRP1) is significantly upregulated in SCs in response to acute injury, activating cJun and promoting SC survival. Matrix-metalloproteinase-9 (MMP-9) is an LRP1 ligand that binds LRP1 through its hemopexin domain (PEX) and activates SC survival signaling and migration. To identify novel peptide mimetics within the hemopexin domain of MMP-9, we examined the crystal structure of PEX, synthesized four peptides, and examined their potential to bind and activate LRP1. We demonstrate that a 22 amino acid peptide, peptide 2, was the only peptide that activated Akt and ERK1/2 signaling in SCs, similar to a glutathione s-transferase (GST)-fused holoprotein, GST-PEX. Intraneural injection of peptide 2, but not vehicle, into crush-injured sciatic nerves activated cJun greater than 2.5-fold in wild-type mice, supporting that peptide 2 can activate the SC repair signaling in vivo. Peptide 2 also bound to Fc-fusion proteins containing the ligand-binding motifs of LRP1, clusters of complement-like repeats (CCRII and CCRIV). Pulldown and computational studies of alanine mutants of peptide 2 showed that positively charged lysine and arginine amino acids within the peptide are critical for stability and binding to CCRII. Collectively, these studies demonstrate that a novel peptide derived from PEX can serve as an LRP1 agonist and possesses qualities previously associated with LRP1 binding and SC signaling in vitro and in vivo.
中文翻译:

使用源自 MMP-9 血红素结构域的肽结合和激活雪旺细胞中 LRP1 依赖性细胞信号传导
雪旺细胞 (SC) 经历表型转化,然后在周围神经系统损伤后协调神经修复。SC 中的低密度脂蛋白受体相关蛋白 1 (LRP1) 在急性损伤后显着上调,激活 cJun 并促进 SC 存活。基质金属蛋白酶-9 (MMP-9) 是一种 LRP1 配体,通过其血红素结合蛋白结构域 (PEX) 结合 LRP1,并激活 SC 生存信号传导和迁移。为了鉴定 MMP-9 血红素结合蛋白结构域内的新型肽模拟物,我们检查了 PEX 的晶体结构,合成了四种肽,并检查了它们结合和激活 LRP1 的潜力。我们证明,22 个氨基酸的肽(肽2)是唯一能在 SC 中激活 Akt 和 ERK1/2 信号传导的肽,类似于谷胱甘肽 s-转移酶 (GST) 融合的全蛋白 GST-PEX。在野生型小鼠中,向挤压损伤的坐骨神经神经内注射肽2 (而非媒介物)激活 cJun 超过 2.5 倍,这支持肽2可以激活体内 SC 修复信号传导。肽2还与含有 LRP1 配体结合基序、补体样重复簇(CCRII 和 CCRIV)的 Fc 融合蛋白结合。肽2的丙氨酸突变体的 Pulldown 和计算研究表明,肽内带正电荷的赖氨酸和精氨酸对于稳定性和与 CCRII 的结合至关重要。总的来说,这些研究表明,一种源自 PEX 的新型肽可以作为 LRP1 激动剂,并具有先前与体外和体内 LRP1 结合和 SC 信号传导相关的特性。
更新日期:2024-03-07
中文翻译:

使用源自 MMP-9 血红素结构域的肽结合和激活雪旺细胞中 LRP1 依赖性细胞信号传导
雪旺细胞 (SC) 经历表型转化,然后在周围神经系统损伤后协调神经修复。SC 中的低密度脂蛋白受体相关蛋白 1 (LRP1) 在急性损伤后显着上调,激活 cJun 并促进 SC 存活。基质金属蛋白酶-9 (MMP-9) 是一种 LRP1 配体,通过其血红素结合蛋白结构域 (PEX) 结合 LRP1,并激活 SC 生存信号传导和迁移。为了鉴定 MMP-9 血红素结合蛋白结构域内的新型肽模拟物,我们检查了 PEX 的晶体结构,合成了四种肽,并检查了它们结合和激活 LRP1 的潜力。我们证明,22 个氨基酸的肽(肽2)是唯一能在 SC 中激活 Akt 和 ERK1/2 信号传导的肽,类似于谷胱甘肽 s-转移酶 (GST) 融合的全蛋白 GST-PEX。在野生型小鼠中,向挤压损伤的坐骨神经神经内注射肽2 (而非媒介物)激活 cJun 超过 2.5 倍,这支持肽2可以激活体内 SC 修复信号传导。肽2还与含有 LRP1 配体结合基序、补体样重复簇(CCRII 和 CCRIV)的 Fc 融合蛋白结合。肽2的丙氨酸突变体的 Pulldown 和计算研究表明,肽内带正电荷的赖氨酸和精氨酸对于稳定性和与 CCRII 的结合至关重要。总的来说,这些研究表明,一种源自 PEX 的新型肽可以作为 LRP1 激动剂,并具有先前与体外和体内 LRP1 结合和 SC 信号传导相关的特性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号