当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vapor–Liquid Equilibrium for Acetonitrile + Ethanol with Imidazole-Based Ionic Liquids as Entrainers at 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-06 , DOI: 10.1021/acs.jced.3c00752 Kun Yue 1 , Jiani Lu 1 , Peiyu Chen 1 , Shaonan Gu 1 , Guowei Zhou 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-06 , DOI: 10.1021/acs.jced.3c00752 Kun Yue 1 , Jiani Lu 1 , Peiyu Chen 1 , Shaonan Gu 1 , Guowei Zhou 1
Affiliation
|
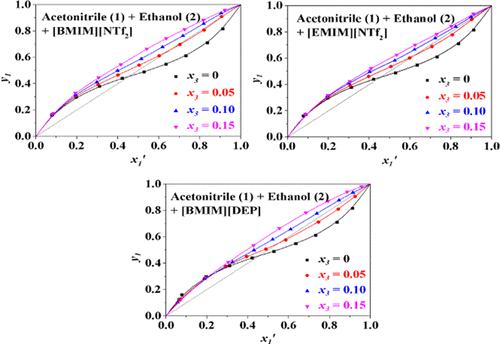
Vapor–liquid equilibrium (VLE) data for the ternary system of acetonitrile + ethanol + ionic liquids (ILs) were obtained at 101.3 kPa, and used ILs were 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([BMIM][NTf2]), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM][NTf2]), and 1-butyl-3-methylimidazolium diethylphosphate ([BMIM][DEP]). The nonrandom two-liquid (NRTL) model was used to correlate the VLE data and fitted well, indicating that the minimum mole fractions of [BMIM][NTf2], [EMIM][NTf2], and [BMIM][DEP] to eliminate the azeotrope was 0.053, 0.063, and 0.065, respectively. These ILs led to an outstanding “salting-in” effect on ethanol, and the salting effect on acetonitrile was inapparent. Higher concentrations of ILs signified a stronger salt effect as well as higher equilibrium temperatures. The results indicated that [BMIM][NTf2], [EMIM][NTf2], and [BMIM][DEP] could be used as good candidate entrainers to separate acetonitrile and ethanol in extractive distillation.
中文翻译:

以咪唑基离子液体为夹带剂的乙腈 + 乙醇在 101.3 kPa 下的汽液平衡
在101.3 kPa下获得乙腈+乙醇+离子液体(ILs)三元体系的气液平衡(VLE)数据,所用ILs为1-丁基-3-甲基咪唑双(三氟甲基磺酰基)亚胺([BMIM][NTf) 2 ])、1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺([EMIM][NTf 2 ])和1-丁基-3-甲基咪唑鎓二乙基磷酸酯([BMIM][DEP])。非随机双液 (NRTL) 模型用于关联 VLE 数据并拟合良好,表明 [BMIM][NTf 2 ]、[EMIM][NTf 2 ] 和 [BMIM][DEP]的最小摩尔分数消除共沸物的效率分别为0.053、0.063和0.065。这些离子液体对乙醇产生显着的“盐析”效应,而对乙腈的盐析效应不明显。 IL 浓度越高意味着盐效应越强以及平衡温度越高。结果表明,[BMIM][NTf 2 ]、[EMIM][NTf 2 ]和[BMIM][DEP]可以作为萃取精馏中分离乙腈和乙醇的良好候选夹带剂。
更新日期:2024-03-06
中文翻译:

以咪唑基离子液体为夹带剂的乙腈 + 乙醇在 101.3 kPa 下的汽液平衡
在101.3 kPa下获得乙腈+乙醇+离子液体(ILs)三元体系的气液平衡(VLE)数据,所用ILs为1-丁基-3-甲基咪唑双(三氟甲基磺酰基)亚胺([BMIM][NTf) 2 ])、1-乙基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺([EMIM][NTf 2 ])和1-丁基-3-甲基咪唑鎓二乙基磷酸酯([BMIM][DEP])。非随机双液 (NRTL) 模型用于关联 VLE 数据并拟合良好,表明 [BMIM][NTf 2 ]、[EMIM][NTf 2 ] 和 [BMIM][DEP]的最小摩尔分数消除共沸物的效率分别为0.053、0.063和0.065。这些离子液体对乙醇产生显着的“盐析”效应,而对乙腈的盐析效应不明显。 IL 浓度越高意味着盐效应越强以及平衡温度越高。结果表明,[BMIM][NTf 2 ]、[EMIM][NTf 2 ]和[BMIM][DEP]可以作为萃取精馏中分离乙腈和乙醇的良好候选夹带剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号