Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Neuronal deletion of Gtf2i results in developmental microglial alterations in a mouse model related to Williams syndrome
Glia ( IF 6.2 ) Pub Date : 2024-03-07 , DOI: 10.1002/glia.24519 Ela Bar 1, 2 , Inbar Fischer 3 , May Rokach 3 , Galit Elad‐Sfadia 1 , Sophie Shirenova 4, 5, 6 , Omer Ophir 3 , Sari Schokoroy Trangle 1 , Eitan Okun 4, 5, 6 , Boaz Barak 1, 3
Glia ( IF 6.2 ) Pub Date : 2024-03-07 , DOI: 10.1002/glia.24519 Ela Bar 1, 2 , Inbar Fischer 3 , May Rokach 3 , Galit Elad‐Sfadia 1 , Sophie Shirenova 4, 5, 6 , Omer Ophir 3 , Sari Schokoroy Trangle 1 , Eitan Okun 4, 5, 6 , Boaz Barak 1, 3
Affiliation
|
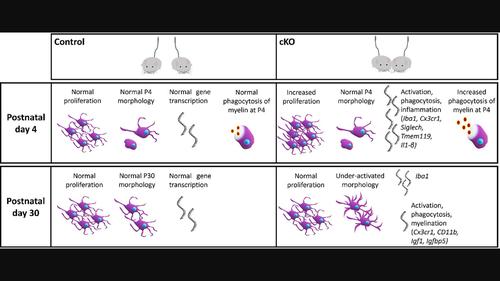
Williams syndrome (WS) is a genetic neurodevelopmental disorder caused by a heterozygous microdeletion, characterized by hypersociability and unique neurocognitive abnormalities. Of the deleted genes, GTF2I has been linked to hypersociability in WS. We have recently shown that Gtf2i deletion from forebrain excitatory neurons, referred to as Gtf2i conditional knockout (cKO) mice leads to multi-faceted myelination deficits associated with the social behaviors affected in WS. These deficits were potentially mediated also by microglia, as they present a close relationship with oligodendrocytes. To study the impact of altered myelination, we characterized these mice in terms of microglia over the course of development. In postnatal day 30 (P30) Gtf2i cKO mice, cortical microglia displayed a more ramified state, as compared with wild type (controls). However, postnatal day 4 (P4) microglia exhibited high proliferation rates and an elevated activation state, demonstrating altered properties related to activation and inflammation in Gtf2i cKO mice compared with control. Intriguingly, P4 Gtf2i cKO-derived microglial cells exhibited significantly elevated myelin phagocytosis in vitro compared to control mice. Lastly, systemic injection of clemastine to P4 Gtf2i cKO and control mice until P30, led to a significant interaction between genotypes and treatments on the expression levels of the phagocytic marker CD68, and a significant reduction of the macrophage/microglial marker Iba1 transcript levels in the cortex of the Gtf2i cKO treated mice. Our data thus implicate microglia as important players in WS, and that early postnatal manipulation of microglia might be beneficial in treating inflammatory and myelin-related pathologies.
中文翻译:

Gtf2i 的神经元缺失导致威廉姆斯综合征相关小鼠模型中小胶质细胞的发育改变
威廉姆斯综合征 (WS) 是一种由杂合性微缺失引起的遗传性神经发育障碍,其特征是过度社交和独特的神经认知异常。在删除的基因中,GTF2I与 WS 的过度社交行为有关。我们最近发现,前脑兴奋性神经元中的Gtf2i缺失(称为Gtf2i条件性敲除 (cKO) 小鼠)会导致与 WS 中受影响的社会行为相关的多方面髓鞘形成缺陷。这些缺陷也可能由小胶质细胞介导,因为它们与少突胶质细胞关系密切。为了研究髓鞘形成改变的影响,我们根据发育过程中的小胶质细胞对这些小鼠进行了表征。在出生后第 30 天 (P30) Gtf2i cKO 小鼠中,与野生型(对照)相比,皮质小胶质细胞表现出更加分支的状态。然而,出生后第 4 天 (P4) 小胶质细胞表现出高增殖率和升高的激活状态,表明与对照组相比, Gtf2i cKO 小鼠与激活和炎症相关的特性发生了改变。有趣的是,与对照小鼠相比,P4 Gtf2i cKO 衍生的小胶质细胞在体外表现出显着升高的髓磷脂吞噬作用。最后,对 P4 Gtf2i cKO 和对照小鼠全身注射氯马斯汀直至 P30,导致基因型和治疗之间对吞噬标记 CD68 表达水平的显着相互作用,并显着降低巨噬细胞/小胶质细胞标记Iba1转录水平。Gtf2i cKO 处理小鼠的皮质。因此,我们的数据表明小胶质细胞是 WS 的重要参与者,并且出生后早期对小胶质细胞的操作可能有益于治疗炎症和髓磷脂相关的病理。
更新日期:2024-03-07
中文翻译:

Gtf2i 的神经元缺失导致威廉姆斯综合征相关小鼠模型中小胶质细胞的发育改变
威廉姆斯综合征 (WS) 是一种由杂合性微缺失引起的遗传性神经发育障碍,其特征是过度社交和独特的神经认知异常。在删除的基因中,GTF2I与 WS 的过度社交行为有关。我们最近发现,前脑兴奋性神经元中的Gtf2i缺失(称为Gtf2i条件性敲除 (cKO) 小鼠)会导致与 WS 中受影响的社会行为相关的多方面髓鞘形成缺陷。这些缺陷也可能由小胶质细胞介导,因为它们与少突胶质细胞关系密切。为了研究髓鞘形成改变的影响,我们根据发育过程中的小胶质细胞对这些小鼠进行了表征。在出生后第 30 天 (P30) Gtf2i cKO 小鼠中,与野生型(对照)相比,皮质小胶质细胞表现出更加分支的状态。然而,出生后第 4 天 (P4) 小胶质细胞表现出高增殖率和升高的激活状态,表明与对照组相比, Gtf2i cKO 小鼠与激活和炎症相关的特性发生了改变。有趣的是,与对照小鼠相比,P4 Gtf2i cKO 衍生的小胶质细胞在体外表现出显着升高的髓磷脂吞噬作用。最后,对 P4 Gtf2i cKO 和对照小鼠全身注射氯马斯汀直至 P30,导致基因型和治疗之间对吞噬标记 CD68 表达水平的显着相互作用,并显着降低巨噬细胞/小胶质细胞标记Iba1转录水平。Gtf2i cKO 处理小鼠的皮质。因此,我们的数据表明小胶质细胞是 WS 的重要参与者,并且出生后早期对小胶质细胞的操作可能有益于治疗炎症和髓磷脂相关的病理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号