Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Short bridging and partial derivatization synergistically modified β-cyclodextrin bonded chiral stationary phases for improved enantioseparation
Talanta ( IF 6.1 ) Pub Date : 2024-03-06 , DOI: 10.1016/j.talanta.2024.125830 Xinyu Liu , Chang Liu , Jianhao Zhou , Xueru Zhao , Youqing Shen , Hailin Cong , Bing Yu
Talanta ( IF 6.1 ) Pub Date : 2024-03-06 , DOI: 10.1016/j.talanta.2024.125830 Xinyu Liu , Chang Liu , Jianhao Zhou , Xueru Zhao , Youqing Shen , Hailin Cong , Bing Yu
|
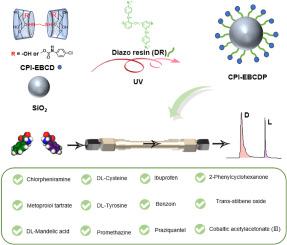
β-Cyclodextrin (β-CD) and its derivatives have been widely employed in the field of chiral separation, but they are still faced the limitation of low enantioselectivity and complex processes. Derivatization with functional molecules or preparation as bridging dimers are the two main modifications for β-CD to obtain chiral recognition compounds. Herein, a partially derived bridged β-CD (CPI-EBCD) bonded chiral stationary phases was prepared to improve enantioseparation. The chiral recognition moiety was synthesized by a bridged β-cyclodextrin dimer using a short-chain bridging agent (ethylenediamine) and then modifying the bridged cyclodextrin with a 4-chlorophenylisocyanate (CPI) containing a benzene ring and polar group. Compared with natural β-CD, dual-chambered CPI-EBCDs have better encapsulation synergies and more recognition sites with the guest molecule, while the short flexible bridging groups make the double cavities closer and more easily recognizable as linear molecules. The introduction of derived groups CPI provided more recognition sites and more types of interactions, including π-π interaction force, hydrogen bonding effect, and dipole-dipole interaction, thus improving the enantiomer-specific chirality recognition effect. The chiral stationary phase CPI-EBCDP was obtained by connecting CPI-EDCB with mesoporous silica microspheres by simple photochemical reaction using a green non-toxic diazo resin as coupling agent, simplifying preparation process. In the reversed phase mode of liquid chromatography, CPI-EBCDP has excellent chiral recognition ability, and 12 chiral compounds are successfully isolated by optimizing mobile phase conditions, with good reproducibility and stability. The successful preparation of this new chiral stationary phase provides an important reference for the subsequent development of cyclodextrin-like chiral stationary phases.
中文翻译:

短桥联和部分衍生化协同修饰的 β-环糊精键合手性固定相可改善对映体分离
β-环糊精(β-CD)及其衍生物已广泛应用于手性分离领域,但仍面临对映选择性低和工艺复杂的限制。功能分子衍生化或制备成桥联二聚体是β-CD获得手性识别化合物的两种主要修饰方式。在此,制备了部分衍生的桥联β-CD(CPI-EBCD)键合手性固定相以改善对映体分离。手性识别部分是通过使用短链桥联剂(乙二胺)合成桥联β-环糊精二聚体,然后用含有苯环和极性基团的4-氯苯基异氰酸酯(CPI)对桥联环糊精进行修饰。与天然β-CD相比,双室CPI-EBCD具有更好的包封协同作用和更多与客体分子的识别位点,而短的柔性桥连基团使双腔更紧密,更容易被识别为线性分子。衍生基团CPI的引入提供了更多的识别位点和更多类型的相互作用,包括π-π相互作用力、氢键效应、偶极-偶极相互作用,从而提高了对映体特异性手性识别效果。以绿色无毒的重氮树脂为偶联剂,通过简单的光化学反应将CPI-EDCB与介孔二氧化硅微球连接,得到手性固定相CPI-EBCDP,简化了制备工艺。在液相色谱反相模式下,CPI-EBCDP具有优异的手性识别能力,通过优化流动相条件成功分离出12种手性化合物,具有良好的重现性和稳定性。这种新型手性固定相的成功制备为后续类环糊精手性固定相的开发提供了重要参考。
更新日期:2024-03-06
中文翻译:

短桥联和部分衍生化协同修饰的 β-环糊精键合手性固定相可改善对映体分离
β-环糊精(β-CD)及其衍生物已广泛应用于手性分离领域,但仍面临对映选择性低和工艺复杂的限制。功能分子衍生化或制备成桥联二聚体是β-CD获得手性识别化合物的两种主要修饰方式。在此,制备了部分衍生的桥联β-CD(CPI-EBCD)键合手性固定相以改善对映体分离。手性识别部分是通过使用短链桥联剂(乙二胺)合成桥联β-环糊精二聚体,然后用含有苯环和极性基团的4-氯苯基异氰酸酯(CPI)对桥联环糊精进行修饰。与天然β-CD相比,双室CPI-EBCD具有更好的包封协同作用和更多与客体分子的识别位点,而短的柔性桥连基团使双腔更紧密,更容易被识别为线性分子。衍生基团CPI的引入提供了更多的识别位点和更多类型的相互作用,包括π-π相互作用力、氢键效应、偶极-偶极相互作用,从而提高了对映体特异性手性识别效果。以绿色无毒的重氮树脂为偶联剂,通过简单的光化学反应将CPI-EDCB与介孔二氧化硅微球连接,得到手性固定相CPI-EBCDP,简化了制备工艺。在液相色谱反相模式下,CPI-EBCDP具有优异的手性识别能力,通过优化流动相条件成功分离出12种手性化合物,具有良好的重现性和稳定性。这种新型手性固定相的成功制备为后续类环糊精手性固定相的开发提供了重要参考。



























 京公网安备 11010802027423号
京公网安备 11010802027423号