当前位置:
X-MOL 学术
›
Forensic Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Application of bioluminescence assay to assess PCR carryover contamination in forensic DNA laboratories
Forensic Chemistry ( IF 2.7 ) Pub Date : 2024-03-03 , DOI: 10.1016/j.forc.2024.100566 Tetsuya Satoh , Yukinobu Kutsuwada , Shota Inokuchi , Takenori Ishida , Takeshi Ikeda , Ryuichi Hirota , Akio Kuroda , Kazutoshi Matsumura , Susumu Iwase
Forensic Chemistry ( IF 2.7 ) Pub Date : 2024-03-03 , DOI: 10.1016/j.forc.2024.100566 Tetsuya Satoh , Yukinobu Kutsuwada , Shota Inokuchi , Takenori Ishida , Takeshi Ikeda , Ryuichi Hirota , Akio Kuroda , Kazutoshi Matsumura , Susumu Iwase
|
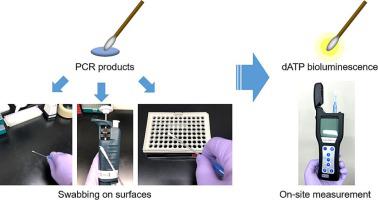
In forensic DNA testing, PCR-based multilocus short tandem repeat (STR) profiling kits, which have high sensitivity and discriminatory power, are generally used to analyze autosomal and Y-chromosomal DNA profiles. Forensic DNA laboratories require strict quality control for DNA testing, as contamination during analyses leads to incorrect interpretation of DNA profiles. Here, we aimed to apply bioluminescence assay to detect and monitor residual PCR products on laboratory work area and equipment surfaces by targeting dATP in the PCR product and allelic ladder marker. Two commercially available bioluminescence assay kits (CheckLite HS Plus and UltraSnap™) were examined for their sensitivity after confirming their reactivity to dATP. In the assay using CheckLite HS Plus, the lower detectable sample volumes were calculated as 10 pl of PCR product of GlobalFiler and PowerPlex Fusion and 1 pl of PCR product of Yfiler Plus and the allelic ladder marker of GlobalFiler, whereas those in the assay using UltraSnap™ were calculated as 1 nl of PCR product and allelic ladder marker. The sample volumes of these kits were lower than those detected through electrophoresis. Thus, the sensitivity of these kits was sufficient to control PCR carryover contamination in the post-PCR areas. Furthermore, residual PCR products in the post-PCR areas were continuously monitored using a bioluminescence assay. The results showed that the bioluminescence values increased after handling PCR samples for electrophoresis and decreased after decontamination. Therefore, we concluded that the bioluminescence assay is useful for assessing PCR carryover contamination in post-PCR processes in forensic DNA laboratories.
中文翻译:

应用生物发光测定法评估法医 DNA 实验室中 PCR 残留污染
在法医DNA检测中,基于PCR的多位点短串联重复(STR)分析试剂盒具有高灵敏度和区分力,通常用于分析常染色体和Y染色体DNA谱。法医 DNA 实验室需要对 DNA 测试进行严格的质量控制,因为分析过程中的污染会导致对 DNA 图谱的错误解读。在这里,我们的目的是通过针对 PCR 产物和等位基因梯标记中的 dATP,应用生物发光测定法来检测和监测实验室工作区域和设备表面上残留的 PCR 产物。在确认两种市售生物发光检测试剂盒(CheckLite HS Plus 和 UltraSnap™)与 dATP 的反应性后,检查了它们的灵敏度。在使用 CheckLite HS Plus 的测定中,较低的可检测样品体积计算为 10 pl GlobalFiler 和 PowerPlex Fusion 的 PCR 产物以及 1 pl Yfiler Plus 的 PCR 产物和 GlobalFiler 的等位基因梯标记,而在使用 UltraSnap 的测定中™ 计算为 1 nl PCR 产物和等位基因梯标记。这些试剂盒的样品量低于通过电泳检测的样品量。因此,这些试剂盒的灵敏度足以控制 PCR 后区域的 PCR 残留污染。此外,使用生物发光测定连续监测 PCR 后区域中残留的 PCR 产物。结果表明,PCR样本电泳后生物发光值升高,去污后生物发光值降低。因此,我们得出结论,生物发光测定可用于评估法医 DNA 实验室 PCR 后过程中的 PCR 残留污染。
更新日期:2024-03-03
中文翻译:

应用生物发光测定法评估法医 DNA 实验室中 PCR 残留污染
在法医DNA检测中,基于PCR的多位点短串联重复(STR)分析试剂盒具有高灵敏度和区分力,通常用于分析常染色体和Y染色体DNA谱。法医 DNA 实验室需要对 DNA 测试进行严格的质量控制,因为分析过程中的污染会导致对 DNA 图谱的错误解读。在这里,我们的目的是通过针对 PCR 产物和等位基因梯标记中的 dATP,应用生物发光测定法来检测和监测实验室工作区域和设备表面上残留的 PCR 产物。在确认两种市售生物发光检测试剂盒(CheckLite HS Plus 和 UltraSnap™)与 dATP 的反应性后,检查了它们的灵敏度。在使用 CheckLite HS Plus 的测定中,较低的可检测样品体积计算为 10 pl GlobalFiler 和 PowerPlex Fusion 的 PCR 产物以及 1 pl Yfiler Plus 的 PCR 产物和 GlobalFiler 的等位基因梯标记,而在使用 UltraSnap 的测定中™ 计算为 1 nl PCR 产物和等位基因梯标记。这些试剂盒的样品量低于通过电泳检测的样品量。因此,这些试剂盒的灵敏度足以控制 PCR 后区域的 PCR 残留污染。此外,使用生物发光测定连续监测 PCR 后区域中残留的 PCR 产物。结果表明,PCR样本电泳后生物发光值升高,去污后生物发光值降低。因此,我们得出结论,生物发光测定可用于评估法医 DNA 实验室 PCR 后过程中的 PCR 残留污染。



























 京公网安备 11010802027423号
京公网安备 11010802027423号