当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activation of Small Molecules by Modified Dodecaborate Anions
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-08 , DOI: 10.1021/acs.jpca.3c07361 Mehmet Emin Kilic 1 , Puru Jena 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-08 , DOI: 10.1021/acs.jpca.3c07361 Mehmet Emin Kilic 1 , Puru Jena 1
Affiliation
|
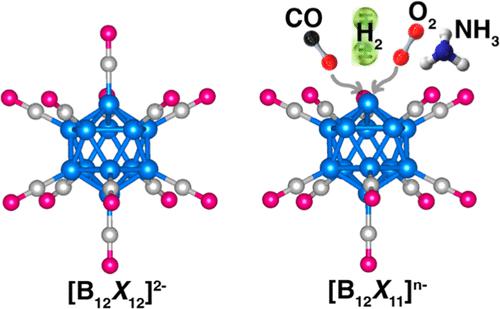
Two of the basic requirements of a good catalyst are that molecules be bound to it with energies intermediate between physisorption and chemisorption and be simultaneously activated in the process. Using density functional theory, we have studied the interaction of small molecules such as H2, O2, N2, CO2, CO, and NH3 with modified dodecaborate anion [B12H12]2–, namely, [B12X11]− and [B12X11]2– (X = H, F, CN). Calculations of the structure, stability, and electronic properties of these species interacting with the above molecules show that they meet the above requirements. In addition, [B12X11]2– (X = F, CN) species are not only more stable than [B12X11]− species but also bind to O2 more strongly than their monoanion counterparts.
中文翻译:

改性十二硼酸根阴离子活化小分子
良好催化剂的两个基本要求是分子以介于物理吸附和化学吸附之间的能量与其结合,并在此过程中同时被激活。利用密度泛函理论,我们研究了H 2、O 2、N 2、CO 2、CO和NH 3等小分子与修饰的十二硼酸阴离子[B 12 H 12 ] 2–的相互作用,即[B 12 X 11 ] -和 [B 12 X 11 ] 2– (X = H、F、CN)。对与上述分子相互作用的这些物质的结构、稳定性和电子性质的计算表明它们满足上述要求。此外,[B 12 X 11 ] 2– (X = F, CN) 物质不仅比 [B 12 X 11 ] -物质更稳定,而且与 O 2的结合比其单阴离子对应物更强。
更新日期:2024-03-08
中文翻译:

改性十二硼酸根阴离子活化小分子
良好催化剂的两个基本要求是分子以介于物理吸附和化学吸附之间的能量与其结合,并在此过程中同时被激活。利用密度泛函理论,我们研究了H 2、O 2、N 2、CO 2、CO和NH 3等小分子与修饰的十二硼酸阴离子[B 12 H 12 ] 2–的相互作用,即[B 12 X 11 ] -和 [B 12 X 11 ] 2– (X = H、F、CN)。对与上述分子相互作用的这些物质的结构、稳定性和电子性质的计算表明它们满足上述要求。此外,[B 12 X 11 ] 2– (X = F, CN) 物质不仅比 [B 12 X 11 ] -物质更稳定,而且与 O 2的结合比其单阴离子对应物更强。



























 京公网安备 11010802027423号
京公网安备 11010802027423号