Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-11 , DOI: 10.3762/bjoc.20.49 Yasuhiro Oishi , Motoharu Kitatani , Koichi Kusakabe
Abstract
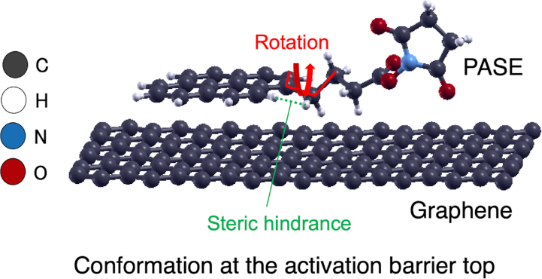
We theoretically analyze possible multiple conformations of protein molecules immobilized by 1-pyrenebutanoic acid succinimidyl ester (PASE) linkers on graphene. The activation barrier between two bi-stable conformations exhibited by PASE is confirmed to be based on the steric hindrance effect between a hydrogen on the pyrene group and a hydrogen on the alkyl group of this molecule. Even after the protein is supplemented, this steric hindrance effect remains if the local structure of the linker consisting of an alkyl group and a pyrene group is maintained. Therefore, it is likely that the kinetic behavior of a protein immobilized with a single PASE linker exhibits an activation barrier-type energy surface between the bi-stable conformations on graphene. We discuss the expected protein sensors when this type of energy surface appears and provide a guideline for improving the sensitivity, especially as an oscillator-type biosensor.
Beilstein J. Org. Chem. 2024, 20, 570–577. doi:10.3762/bjoc.20.49
中文翻译:

石墨烯上吸附的芘丁酸连接的蛋白质分子可能的双稳态结构:理论研究
摘要
我们从理论上分析了石墨烯上 1-芘丁酸琥珀酰亚胺酯 (PASE) 连接体固定的蛋白质分子可能的多种构象。PASE所表现出的两个双稳态构象之间的活化势垒被证实是基于该分子芘基上的氢和烷基上的氢之间的空间位阻效应。即使补充蛋白质后,如果维持由烷基和芘基团组成的连接体的局部结构,则这种空间位阻效应仍然存在。因此,用单个 PASE 连接体固定的蛋白质的动力学行为很可能在石墨烯上的双稳态构象之间表现出活化势垒型能量表面。我们讨论了当这种类型的能量表面出现时预期的蛋白质传感器,并提供了提高灵敏度的指南,特别是作为振荡器型生物传感器。

贝尔斯坦 J. 组织。化学。 2024, 20, 570–577。doi:10.3762/bjoc.20.49



























 京公网安备 11010802027423号
京公网安备 11010802027423号