Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-11 , DOI: 10.3762/bjoc.20.48 Alexander Yanovich , Anastasia Vepreva , Ksenia Malkova , Grigory Kantin , Dmitry Dar’in
Abstract
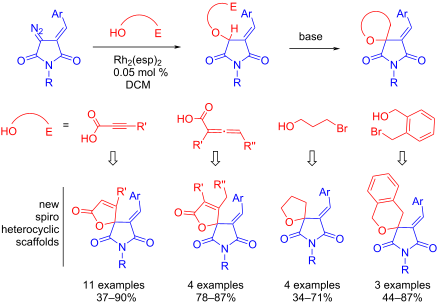
A facile approach to novel medicinally relevant spiro heterocyclic scaffolds (namely furan-2(5H)-ones, tetrahydrofurans and pyrans spiro-conjugated with the succinimide ring) has been developed. The protocol consists of Rh(II)-catalyzed insertion of heterocyclic carbenes derived from diazoarylidene succinimides (DAS) into the O–H bond of propiolic/allenic acids or brominated alcohols, followed by base-promoted cyclization to afford the target spirocyclic compounds in good to high yields.
Beilstein J. Org. Chem. 2024, 20, 561–569. doi:10.3762/bjoc.20.48
中文翻译:

通过串联 Rh(II) 催化的 O–H 插入/碱基促进环化(涉及重氮亚芳基琥珀酰亚胺)进入新的螺杂环
摘要
已经开发出一种简便的方法来制备新型医学相关的螺杂环支架(即与琥珀酰亚胺环共轭的呋喃-2(5H ) -酮、四氢呋喃和吡喃螺环)。该方案包括 Rh(II) 催化将源自重氮亚芳基琥珀酰亚胺 (DAS) 的杂环卡宾插入丙炔酸/丙二烯酸或溴化醇的 O-H 键中,然后进行碱促进环化,以良好的方式提供目标螺环化合物。达到高产量。

贝尔斯坦 J. 组织。化学。 2024, 20, 561–569。doi:10.3762/bjoc.20.48



























 京公网安备 11010802027423号
京公网安备 11010802027423号