当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural enzymology studies with the substrate 3S-hydroxybutanoyl-CoA: bifunctional MFE1 is a less efficient dehydrogenase than monofunctional HAD
FEBS Open Bio ( IF 2.6 ) Pub Date : 2024-03-08 , DOI: 10.1002/2211-5463.13786 Shruthi Sridhar 1 , Tiila‐Riikka Kiema 2 , Werner Schmitz 3 , Mikael Widersten 4 , Rik K. Wierenga 1
FEBS Open Bio ( IF 2.6 ) Pub Date : 2024-03-08 , DOI: 10.1002/2211-5463.13786 Shruthi Sridhar 1 , Tiila‐Riikka Kiema 2 , Werner Schmitz 3 , Mikael Widersten 4 , Rik K. Wierenga 1
Affiliation
|
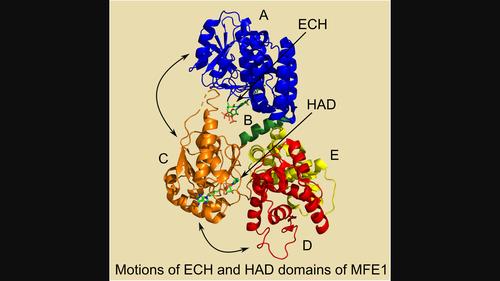
Multifunctional enzyme, type-1 (MFE1) catalyzes the second and third step of the β-oxidation cycle, being, respectively, the 2E-enoyl-CoA hydratase (ECH) reaction (N-terminal part, crotonase fold) and the NAD+-dependent, 3S-hydroxyacyl-CoA dehydrogenase (HAD) reaction (C-terminal part, HAD fold). Structural enzymological properties of rat MFE1 (RnMFE1) as well as of two of its variants, namely the E123A variant (a glutamate of the ECH active site is mutated into alanine) and the BCDE variant (without domain A of the ECH part), were studied, using as substrate 3S-hydroxybutanoyl-CoA. Protein crystallographic binding studies show the hydrogen bond interactions of 3S-hydroxybutanoyl-CoA as well as of its 3-keto, oxidized form, acetoacetyl-CoA, with the catalytic glutamates in the ECH active site. Pre-steady state binding experiments with NAD+ and NADH show that the kon and koff rate constants of the HAD active site of monomeric RnMFE1 and the homologous human, dimeric 3S-hydroxyacyl-CoA dehydrogenase (HsHAD) for NAD+ and NADH are very similar, being the same as those observed for the E123A and BCDE variants. However, steady state and pre-steady state kinetic data concerning the HAD-catalyzed dehydrogenation reaction of the substrate 3S-hydroxybutanoyl-CoA show that, respectively, the kcat and kchem rate constants for conversion into acetoacetyl-CoA by RnMFE1 (and its two variants) are about 10 fold lower as when catalyzed by HsHAD. The dynamical properties of dehydrogenases are known to be important for their catalytic efficiency, and it is discussed that the greater complexity of the RnMFE1 fold correlates with the observation that RnMFE1 is a slower dehydrogenase than HsHAD.
中文翻译:

使用底物 3S-羟基丁酰基-CoA 进行结构酶学研究:双功能 MFE1 的脱氢酶效率低于单功能 HAD
多功能酶,1 型 (MFE1) 催化 β-氧化循环的第二步和第三步,分别是 2 E -烯酰辅酶 A 水合酶 (ECH) 反应(N 末端部分,巴豆酶折叠)和 NAD + -依赖性,3S-羟酰基辅酶A脱氢酶(HAD)反应(C末端部分,HAD折叠)。大鼠 MFE1 (RnMFE1) 及其两个变体,即 E123A 变体(ECH 活性位点的谷氨酸突变为丙氨酸)和 BCDE 变体(ECH 部分没有结构域 A)的结构酶学特性研究使用3S-羟基丁酰辅酶A作为底物。蛋白质晶体学结合研究表明3S-羟基丁酰基-CoA 及其 3-酮氧化形式乙酰乙酰基-CoA 与 ECH 活性位点中的催化谷氨酸盐存在氢键相互作用。 NAD +和 NADH的预稳态结合实验表明, NAD +和 NADH的单体 RnMFE1 的 HAD 活性位点和同源人二聚体 3S -羟酰基辅酶 A 脱氢酶 (HsHAD)的k on和k off速率常数非常相似,与 E123A 和 BCDE 变体观察到的结果相同。然而,关于底物3S-羟基丁酰基-CoA的HAD催化脱氢反应的稳态和预稳态动力学数据分别表明, RnMFE1(和它的两种变体)比 HsHAD 催化时低约 10 倍。已知脱氢酶的动力学特性对其催化效率很重要,并且讨论了 RnMFE1 折叠的更大复杂性与 RnMFE1 是比 HsHAD 更慢的脱氢酶的观察结果相关。
更新日期:2024-03-08
中文翻译:

使用底物 3S-羟基丁酰基-CoA 进行结构酶学研究:双功能 MFE1 的脱氢酶效率低于单功能 HAD
多功能酶,1 型 (MFE1) 催化 β-氧化循环的第二步和第三步,分别是 2 E -烯酰辅酶 A 水合酶 (ECH) 反应(N 末端部分,巴豆酶折叠)和 NAD + -依赖性,3S-羟酰基辅酶A脱氢酶(HAD)反应(C末端部分,HAD折叠)。大鼠 MFE1 (RnMFE1) 及其两个变体,即 E123A 变体(ECH 活性位点的谷氨酸突变为丙氨酸)和 BCDE 变体(ECH 部分没有结构域 A)的结构酶学特性研究使用3S-羟基丁酰辅酶A作为底物。蛋白质晶体学结合研究表明3S-羟基丁酰基-CoA 及其 3-酮氧化形式乙酰乙酰基-CoA 与 ECH 活性位点中的催化谷氨酸盐存在氢键相互作用。 NAD +和 NADH的预稳态结合实验表明, NAD +和 NADH的单体 RnMFE1 的 HAD 活性位点和同源人二聚体 3S -羟酰基辅酶 A 脱氢酶 (HsHAD)的k on和k off速率常数非常相似,与 E123A 和 BCDE 变体观察到的结果相同。然而,关于底物3S-羟基丁酰基-CoA的HAD催化脱氢反应的稳态和预稳态动力学数据分别表明, RnMFE1(和它的两种变体)比 HsHAD 催化时低约 10 倍。已知脱氢酶的动力学特性对其催化效率很重要,并且讨论了 RnMFE1 折叠的更大复杂性与 RnMFE1 是比 HsHAD 更慢的脱氢酶的观察结果相关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号