当前位置:
X-MOL 学术
›
J. Chin. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Condensed Fukui function and experimental evaluation of the corrosion inhibition properties of some antipyrinyl-imidazotriazole and their derivatives for copper in an acidic environment
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2024-03-11 , DOI: 10.1002/jccs.202300351 Shymaa Adel Elsayed 1 , Nehal A. Barghout 1 , Safaa Ragab 1 , Ehab Abdel‐Latif 2 , Hassan Ali Etman 2 , Mohamed A. Hamed 1 , Nnabuk Okon Eddy 3 , Ahmed El Nemr 1
Journal of the Chinese Chemical Society ( IF 1.8 ) Pub Date : 2024-03-11 , DOI: 10.1002/jccs.202300351 Shymaa Adel Elsayed 1 , Nehal A. Barghout 1 , Safaa Ragab 1 , Ehab Abdel‐Latif 2 , Hassan Ali Etman 2 , Mohamed A. Hamed 1 , Nnabuk Okon Eddy 3 , Ahmed El Nemr 1
Affiliation
|
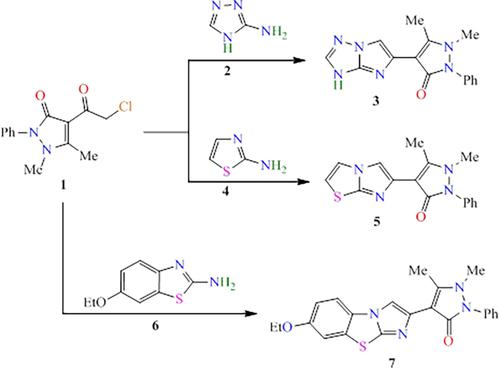
The effectiveness of synthesized antipyrinyl-imidazotriazole and its derivatives as inhibitors for the corrosion of copper alloy in 0.5 M H2SO4 solution was tested using weight loss, electrochemical impedance spectroscopy (EIS) and potentiodyanmic polarization techniques. The generated results confirmed that the tested compounds have strong inhibition efficiencies for the protection of the corrosion of copper alloy in 0.5 M H2SO4. Maximum inhibition efficiencies (IEs) evaluated from electrochemical measurements at inhibitor's concentrations of 0.040 g/L were 85% (5-(4-Antipyrinyl)-3H-imidazo[1,2-b][1,2,4]triazole), 60% (6-Antipyrinyl-imidazo[2,1-b]thiazole) and 72% (2-Antipyrinyl-7-ethoxy-imidazo[2,1-b]benzothiazole). It was observed that the inhibition efficiency was strongly influenced by the flow rate of the solution and was reduced to 79 (5-(4-Antipyrinyl)-3H-imidazo[1,2-b][1,2,4]triazole), 60% (6-Antipyrinyl-imidazo[2,1-b]thiazole), and 44% (2-Antipyrinyl-7-ethoxy-imidazo[2,1-b]benzothiazole) at an agitation speed of 400 rpm. EI from weight loss was comparable with those from PDP of mixed-type inhibition style and EIS of diffusion model. The low inhibition efficiency for 6-Antipyrinyl-imidazo[2,1-b]thiazole was significantly enhanced from 60% to 90% through a synergistic effect of 0.0001 M KI. The Temkin and Frumkin isotherms indicate the physical adsorption of inhibitors on copper surface. Condensed Fukui function calculations reveal a common center for electrophilic attacks in the three molecules: the nitrogen in the bridged pyrrole rings (labeled as N11).
中文翻译:

酸性环境下安替比林咪唑三唑及其衍生物对铜缓蚀性能的凝聚福井函数及实验评价
采用失重、电化学阻抗谱(EIS)和动电位极化技术测试了合成的安替比林咪唑三唑及其衍生物作为铜合金在0.5 MH 2 SO 4溶液中的缓蚀剂的有效性。结果表明,所测试的化合物对于铜合金在0.5 MH 2 SO 4中的腐蚀具有很强的缓蚀效果。在抑制剂浓度为 0.040 g/L 时通过电化学测量评估的最大抑制效率 (IE) 为 85% (5-(4-安替比林)-3 H-咪唑并[1,2- b ][1,2,4]三唑) ,60%(6-安替比林基-咪唑并[2,1- b ]噻唑)和72%(2-安替比林基-7-乙氧基-咪唑[2,1- b ]苯并噻唑)。据观察,抑制效率受溶液流速的强烈影响,并降低至 79 (5-(4-安替比林基)-3 H-咪唑并[1,2- b ][1,2,4]三唑)、60%(6-安替比林基-咪唑并[2,1- b ]噻唑)和44%(2-安替比林基-7-乙氧基-咪唑[2,1- b ]苯并噻唑),搅拌速度为400 rpm。减重的 EI 与混合型抑制型 PDP 和扩散模型的 EIS 相当。通过0.0001 M KI的协同作用, 6-安替比林基-咪唑并[2,1- b ]噻唑的低抑制效率从60%显着提高到90%。 Temkin 和 Frumkin 等温线表明抑制剂在铜表面的物理吸附。凝聚福井函数计算揭示了三个分子中亲电子攻击的共同中心:桥联吡咯环中的氮(标记为 N11)。
更新日期:2024-03-11
中文翻译:

酸性环境下安替比林咪唑三唑及其衍生物对铜缓蚀性能的凝聚福井函数及实验评价
采用失重、电化学阻抗谱(EIS)和动电位极化技术测试了合成的安替比林咪唑三唑及其衍生物作为铜合金在0.5 MH 2 SO 4溶液中的缓蚀剂的有效性。结果表明,所测试的化合物对于铜合金在0.5 MH 2 SO 4中的腐蚀具有很强的缓蚀效果。在抑制剂浓度为 0.040 g/L 时通过电化学测量评估的最大抑制效率 (IE) 为 85% (5-(4-安替比林)-3 H-咪唑并[1,2- b ][1,2,4]三唑) ,60%(6-安替比林基-咪唑并[2,1- b ]噻唑)和72%(2-安替比林基-7-乙氧基-咪唑[2,1- b ]苯并噻唑)。据观察,抑制效率受溶液流速的强烈影响,并降低至 79 (5-(4-安替比林基)-3 H-咪唑并[1,2- b ][1,2,4]三唑)、60%(6-安替比林基-咪唑并[2,1- b ]噻唑)和44%(2-安替比林基-7-乙氧基-咪唑[2,1- b ]苯并噻唑),搅拌速度为400 rpm。减重的 EI 与混合型抑制型 PDP 和扩散模型的 EIS 相当。通过0.0001 M KI的协同作用, 6-安替比林基-咪唑并[2,1- b ]噻唑的低抑制效率从60%显着提高到90%。 Temkin 和 Frumkin 等温线表明抑制剂在铜表面的物理吸附。凝聚福井函数计算揭示了三个分子中亲电子攻击的共同中心:桥联吡咯环中的氮(标记为 N11)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号