当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
NIR-Active Gold Dogbone Nanorattles Impregnated in Cationic Dextrin Nanoparticles for Cancer Nanotheranostics
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2024-03-11 , DOI: 10.1021/acsbiomaterials.3c01176 Ankita Sarkar 1 , Khushal Singh 1 , Keshav Bhardwaj 1 , Amit Jaiswal 1
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2024-03-11 , DOI: 10.1021/acsbiomaterials.3c01176 Ankita Sarkar 1 , Khushal Singh 1 , Keshav Bhardwaj 1 , Amit Jaiswal 1
Affiliation
|
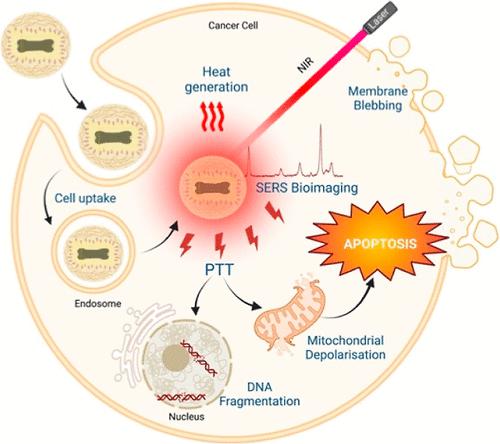
Theranostic systems, which integrate therapy and diagnosis into a single platform, have gained significant attention as a promising approach for noninvasive cancer treatment. The field of image-guided therapy has revolutionized real-time tumor detection, and within this domain, plasmonic nanostructures have garnered significant attention. These structures possess unique localized surface plasmon resonance (LSPR), allowing for enhanced absorption in the near-infrared (NIR) range. By leveraging the heat generated from plasmonic nanoparticles upon NIR irradiation, target cancer cells can be effectively eradicated. This study introduces a plasmonic gold dogbone-nanorattle (AuDB NRT) structure that exhibits broad absorption in the NIR region and demonstrates a photothermal conversion efficiency of 35.29%. When exposed to an NIR laser, the AuDB NRTs generate heat, achieving a maximum temperature rise of 38 °C at a concentration of 200 μg/mL and a laser power density of 3 W/cm2. Additionally, the AuDB NRTs possess intrinsic electromagnetic hotspots that amplify the signal of a Raman reporter molecule, making them an excellent probe for surface-enhanced Raman scattering-based bioimaging of cancer cells. To improve the biocompatibility of the nanorattles, the AuDB NRTs were conjugated with mPEG-thiol and successfully encapsulated into cationic dextrin nanoparticles (CD NPs). Biocompatibility tests were performed on HEK 293 A and MCF-7 cell lines, revealing high cell viability when exposed to AuDB NRT-CD NPs. Remarkably, even at a low laser power density of 1 W/cm2, the application of the NIR laser resulted in a remarkable 80% cell death in cells treated with a nanocomposite concentration of 100 μg/mL. Further investigation elucidated that the cell death induced by photothermal heat followed an apoptotic mechanism. Overall, our findings highlight the significant potential of the prepared nanocomposite for cancer theranostics, combining effective photothermal therapy along with the ability to image cancer cells.
中文翻译:

浸渍在阳离子糊精纳米颗粒中的近红外活性金狗骨纳米摇铃用于癌症纳米治疗学
治疗诊断系统将治疗和诊断集成到一个平台中,作为一种有前景的非侵入性癌症治疗方法而受到广泛关注。图像引导治疗领域彻底改变了实时肿瘤检测,在该领域中,等离子体纳米结构引起了广泛关注。这些结构具有独特的局域表面等离子体共振(LSPR),可以增强近红外(NIR)范围内的吸收。通过利用近红外辐射下等离子体纳米粒子产生的热量,可以有效地根除目标癌细胞。这项研究引入了一种等离子体金狗骨纳米片 (AuDB NRT) 结构,该结构在近红外区域表现出广泛的吸收,并表现出 35.29% 的光热转换效率。当暴露于近红外激光时,AuDB NRT 会产生热量,在浓度为 200 μg/mL 和激光功率密度为 3 W/cm 2时实现最大温升 38 °C 。此外,AuDB NRT 具有内在的电磁热点,可以放大拉曼报告分子的信号,使其成为基于表面增强拉曼散射的癌细胞生物成像的优秀探针。为了提高纳米摇铃的生物相容性,将 AuDB NRT 与 mPEG-硫醇缀合,并成功封装到阳离子糊精纳米颗粒 (CD NP) 中。对 HEK 293 A 和 MCF-7 细胞系进行生物相容性测试,结果表明暴露于 AuDB NRT-CD NP 时细胞具有高活力。值得注意的是,即使在 1 W/cm 2的低激光功率密度下,NIR 激光的应用也导致用 100 μg/mL 纳米复合材料浓度处理的细胞显着 80% 的细胞死亡。进一步的研究表明,光热诱导的细胞死亡遵循细胞凋亡机制。总的来说,我们的研究结果强调了所制备的纳米复合材料在癌症治疗诊断方面的巨大潜力,它将有效的光热疗法与癌细胞成像的能力结合起来。
更新日期:2024-03-11
中文翻译:

浸渍在阳离子糊精纳米颗粒中的近红外活性金狗骨纳米摇铃用于癌症纳米治疗学
治疗诊断系统将治疗和诊断集成到一个平台中,作为一种有前景的非侵入性癌症治疗方法而受到广泛关注。图像引导治疗领域彻底改变了实时肿瘤检测,在该领域中,等离子体纳米结构引起了广泛关注。这些结构具有独特的局域表面等离子体共振(LSPR),可以增强近红外(NIR)范围内的吸收。通过利用近红外辐射下等离子体纳米粒子产生的热量,可以有效地根除目标癌细胞。这项研究引入了一种等离子体金狗骨纳米片 (AuDB NRT) 结构,该结构在近红外区域表现出广泛的吸收,并表现出 35.29% 的光热转换效率。当暴露于近红外激光时,AuDB NRT 会产生热量,在浓度为 200 μg/mL 和激光功率密度为 3 W/cm 2时实现最大温升 38 °C 。此外,AuDB NRT 具有内在的电磁热点,可以放大拉曼报告分子的信号,使其成为基于表面增强拉曼散射的癌细胞生物成像的优秀探针。为了提高纳米摇铃的生物相容性,将 AuDB NRT 与 mPEG-硫醇缀合,并成功封装到阳离子糊精纳米颗粒 (CD NP) 中。对 HEK 293 A 和 MCF-7 细胞系进行生物相容性测试,结果表明暴露于 AuDB NRT-CD NP 时细胞具有高活力。值得注意的是,即使在 1 W/cm 2的低激光功率密度下,NIR 激光的应用也导致用 100 μg/mL 纳米复合材料浓度处理的细胞显着 80% 的细胞死亡。进一步的研究表明,光热诱导的细胞死亡遵循细胞凋亡机制。总的来说,我们的研究结果强调了所制备的纳米复合材料在癌症治疗诊断方面的巨大潜力,它将有效的光热疗法与癌细胞成像的能力结合起来。



























 京公网安备 11010802027423号
京公网安备 11010802027423号