当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical Insights into the Gas-Phase Oxidation of 3-Methyl-2-butene-1-thiol by the OH Radical: Thermochemical and Kinetic Analysis
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.jpca.3c07775 Parandaman Arathala 1 , Rabi A. Musah 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-11 , DOI: 10.1021/acs.jpca.3c07775 Parandaman Arathala 1 , Rabi A. Musah 1
Affiliation
|
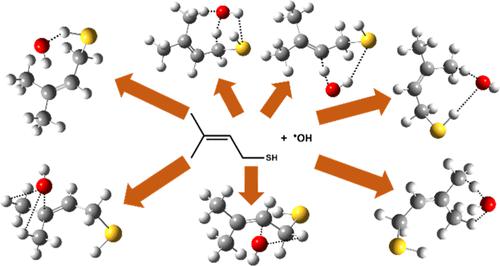
3-Methyl-2-butene-1-thiol ((CH3)2C═CH–CH2–SH; MBT) is a recently identified volatile organosulfur compound emitted from Cannabis sativa and is purported to contribute to its skunky odor. To understand its environmental fate, hydroxyl radical (•OH)-mediated oxidation of MBT was conducted using high-level quantum chemical and theoretical kinetic calculations. Three stable conformers were identified for the title molecule. Abstraction and addition pathways are possible for the MBT + OH radical reaction, and thus, potential energy surfaces involving H-abstraction and •OH addition were computed at the CCSD(T)/aug-cc-pV(T+d)Z//M06-2X/aug-cc-pV(T+d)Z level of theory. The barrier height for the addition of the OH radical to a C atom of the alkene moiety, leading to the formation of a C-centered MBT–OH radical, was computed to be −4.1 kcal mol–1 below the energy of the starting MBT + OH radical-separated reactants. This reaction was found to be dominant compared to other site-specific H-abstraction and addition paths. The kinetics of all the site-specific abstraction and addition reactions associated with the most stable MBT + OH radical reaction were assessed using the MESMER kinetic code between 200 and 320 K. Further, we considered the contributions from two other conformers of MBT to the overall reaction of MBT + OH radical. The estimated global rate coefficient for the oxidation of MBT with respect to its reactions with the OH radical was found to be 6.1 × 10–11 cm3 molecule–1 s–1 at 298 K and 1 atm pressure. The thermodynamic parameters and atmospheric implications of the MBT + OH reaction are discussed.
中文翻译:

OH 自由基气相氧化 3-甲基-2-丁烯-1-硫醇的理论见解:热化学和动力学分析
3-甲基-2-丁烯-1-硫醇 ((CH 3 ) 2 C=CH–CH 2 –SH; MBT) 是最近发现的一种从大麻中释放出的挥发性有机硫化合物,据称是造成其臭味的原因。为了了解其环境归趋,使用高级量子化学和理论动力学计算进行了羟基自由基 ( · OH) 介导的 MBT 氧化。鉴定出标题分子的三个稳定构象异构体。 MBT + OH 自由基反应可能存在抽象和加成途径,因此,在 CCSD(T)/aug-cc-pV(T+d)Z// 处计算了涉及 H 抽象和• OH 加成的势能面M06-2X/aug-cc-pV(T+d)Z 理论水平。 OH 自由基加成到烯烃部分的 C 原子上(导致形成以 C 为中心的 MBT-OH 自由基)的势垒高度经计算为 -4.1 kcal mol –1 ,低于起始 MBT 的能量+ OH自由基分离的反应物。与其他位点特异性 H 抽象和添加路径相比,该反应被发现占主导地位。使用 200 至 320 K 之间的 MESMER 动力学代码评估了与最稳定的 MBT + OH 自由基反应相关的所有位点特异性抽提和加成反应的动力学。此外,我们还考虑了 MBT 的其他两种构象异构体对整体的贡献。 MBT+OH自由基反应。在 298 K 和 1 atm 压力下,MBT 与 OH 自由基反应的氧化估计总体速率系数为 6.1 × 10 –11 cm 3 molecular –1 s –1 。讨论了 MBT + OH 反应的热力学参数和大气影响。
更新日期:2024-03-11
中文翻译:

OH 自由基气相氧化 3-甲基-2-丁烯-1-硫醇的理论见解:热化学和动力学分析
3-甲基-2-丁烯-1-硫醇 ((CH 3 ) 2 C=CH–CH 2 –SH; MBT) 是最近发现的一种从大麻中释放出的挥发性有机硫化合物,据称是造成其臭味的原因。为了了解其环境归趋,使用高级量子化学和理论动力学计算进行了羟基自由基 ( · OH) 介导的 MBT 氧化。鉴定出标题分子的三个稳定构象异构体。 MBT + OH 自由基反应可能存在抽象和加成途径,因此,在 CCSD(T)/aug-cc-pV(T+d)Z// 处计算了涉及 H 抽象和• OH 加成的势能面M06-2X/aug-cc-pV(T+d)Z 理论水平。 OH 自由基加成到烯烃部分的 C 原子上(导致形成以 C 为中心的 MBT-OH 自由基)的势垒高度经计算为 -4.1 kcal mol –1 ,低于起始 MBT 的能量+ OH自由基分离的反应物。与其他位点特异性 H 抽象和添加路径相比,该反应被发现占主导地位。使用 200 至 320 K 之间的 MESMER 动力学代码评估了与最稳定的 MBT + OH 自由基反应相关的所有位点特异性抽提和加成反应的动力学。此外,我们还考虑了 MBT 的其他两种构象异构体对整体的贡献。 MBT+OH自由基反应。在 298 K 和 1 atm 压力下,MBT 与 OH 自由基反应的氧化估计总体速率系数为 6.1 × 10 –11 cm 3 molecular –1 s –1 。讨论了 MBT + OH 反应的热力学参数和大气影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号