当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetic Modeling Analysis of Ar Addition to Atmospheric Pressure N2–H2 Plasma for Plasma-Assisted Catalytic Synthesis of NH3
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-13 , DOI: 10.1021/acs.jpca.3c06841 Zihan Lin 1 , Shota Abe 1, 2 , Zhe Chen 1 , Surabhi Jaiswal 1 , Bruce E. Koel 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-13 , DOI: 10.1021/acs.jpca.3c06841 Zihan Lin 1 , Shota Abe 1, 2 , Zhe Chen 1 , Surabhi Jaiswal 1 , Bruce E. Koel 1
Affiliation
|
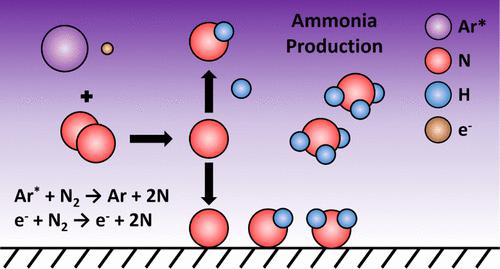
Zero-dimensional kinetic modeling of atmospheric pressure Ar–N2–H2 nonthermal plasma was carried out to gain mechanistic insights into plasma-assisted catalytic synthesis of ammonia. Ar dilution is a common technique for tailoring plasma discharge properties and has been shown to enhance NH3 formation when added to N2–H2 plasma. The kinetic model was developed for a coaxial dielectric barrier discharge quartz wool-packed bed reactor operating at near room temperature using a kHz-frequency plasma source. With 30% Ar mixed in a 1:1 N2–H2 plasma at 760 Torr, we find that NH3 production is dominated by Eley–Rideal (E-R) surface reactions, which heavily involve surface NHx species derived from N and H radicals in the gas phase, while the influence of excited N2 molecules is negligible. This is contrary to the commonly proposed mechanism that excited N2 molecules created by Penning excitation of N2 by Ar(4s) and Ar(4p) play a significant role in assisting NH3 formation. Our model shows that the enhanced NH3 formation upon Ar dilution is unlikely due to the interactions between Ar and H species, as excited Ar atoms have a weak effect on H radical formation through H2 dissociation compared to electrons. We find that excited Ar atoms contribute to 28% of the N radical production in the gas phase via N2 dissociation, while the rest are dominated by electron-impact dissociation. Furthermore, Ar species play a negligible role in the product NH3 dissociation. N2 conversion sensitivity analyses were carried out for electron number density (ne) and reduced electric field (E/N), and contributions from Ar to gas-phase N radical production were quantified. The model can provide guidance on potential reasons for observing enhanced NH3 formation upon Ar dilution in N2–H2 plasma beyond changes in the discharge characteristics.
中文翻译:

常压 N2-H2 等离子体中 Ar 加成等离子体辅助催化合成 NH3 的动力学模型分析
对大气压Ar-N 2 -H 2非热等离子体进行零维动力学建模,以获得等离子体辅助催化氨合成的机理见解。 Ar 稀释是调整等离子体放电特性的常用技术,并且已被证明当添加到 N 2 –H 2等离子体中时可以增强 NH 3 的形成。该动力学模型是针对使用 kHz 频率等离子体源在接近室温下运行的同轴介质阻挡放电石英棉填充床反应器开发的。当 30% Ar在 760 Torr 下混合在 1:1 N 2 –H 2等离子体中时,我们发现 NH 3的产生主要是 Eley-Rideal (ER) 表面反应,其中大量涉及源自 N 和 H 的表面 NH x物质。气相中的自由基,而激发的N 2分子的影响可以忽略不计。这与通常提出的机制相反,即通过 Ar(4s) 和 Ar(4p)潘宁激发 N 2产生的激发 N 2分子在协助 NH 3形成中发挥重要作用。我们的模型表明,由于 Ar 和 H 物质之间的相互作用,Ar 稀释时不太可能增强 NH 3的形成,因为与电子相比,激发的 Ar 原子通过 H 2解离对 H 自由基形成的影响较弱。我们发现,激发的Ar原子通过N 2离解贡献了气相中28%的N自由基产生,而其余部分则主要由电子碰撞离解产生。此外,Ar物质在产物NH 3解离中发挥的作用可以忽略不计。对电子数密度( ne )和还原电场(E/N)进行N 2转换灵敏度分析,并量化Ar对气相N自由基产生的贡献。除了放电特性的变化之外,该模型还可以为观察N 2 –H 2等离子体中 Ar 稀释后NH 3形成增强的潜在原因提供指导。
更新日期:2024-03-13
中文翻译:

常压 N2-H2 等离子体中 Ar 加成等离子体辅助催化合成 NH3 的动力学模型分析
对大气压Ar-N 2 -H 2非热等离子体进行零维动力学建模,以获得等离子体辅助催化氨合成的机理见解。 Ar 稀释是调整等离子体放电特性的常用技术,并且已被证明当添加到 N 2 –H 2等离子体中时可以增强 NH 3 的形成。该动力学模型是针对使用 kHz 频率等离子体源在接近室温下运行的同轴介质阻挡放电石英棉填充床反应器开发的。当 30% Ar在 760 Torr 下混合在 1:1 N 2 –H 2等离子体中时,我们发现 NH 3的产生主要是 Eley-Rideal (ER) 表面反应,其中大量涉及源自 N 和 H 的表面 NH x物质。气相中的自由基,而激发的N 2分子的影响可以忽略不计。这与通常提出的机制相反,即通过 Ar(4s) 和 Ar(4p)潘宁激发 N 2产生的激发 N 2分子在协助 NH 3形成中发挥重要作用。我们的模型表明,由于 Ar 和 H 物质之间的相互作用,Ar 稀释时不太可能增强 NH 3的形成,因为与电子相比,激发的 Ar 原子通过 H 2解离对 H 自由基形成的影响较弱。我们发现,激发的Ar原子通过N 2离解贡献了气相中28%的N自由基产生,而其余部分则主要由电子碰撞离解产生。此外,Ar物质在产物NH 3解离中发挥的作用可以忽略不计。对电子数密度( ne )和还原电场(E/N)进行N 2转换灵敏度分析,并量化Ar对气相N自由基产生的贡献。除了放电特性的变化之外,该模型还可以为观察N 2 –H 2等离子体中 Ar 稀释后NH 3形成增强的潜在原因提供指导。



























 京公网安备 11010802027423号
京公网安备 11010802027423号