当前位置:
X-MOL 学术
›
Cell. Mol. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Endothelial Slc35a1 Deficiency Causes Loss of LSEC Identity and Exacerbates Neonatal Lipid Deposition in the Liver in Mice
Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.jcmgh.2024.03.002 Bin Zuo , Fei Yang , Lulu Huang , Jingjing Han , Tianyi Li , Zhenni Ma , Lijuan Cao , Yun Li , Xia Bai , Miao Jiang , Yang He , Lijun Xia
Cellular and Molecular Gastroenterology and Hepatology ( IF 7.2 ) Pub Date : 2024-03-11 , DOI: 10.1016/j.jcmgh.2024.03.002 Bin Zuo , Fei Yang , Lulu Huang , Jingjing Han , Tianyi Li , Zhenni Ma , Lijuan Cao , Yun Li , Xia Bai , Miao Jiang , Yang He , Lijun Xia
|
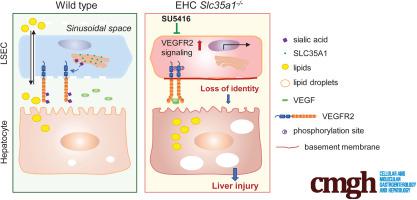
The functional maturation of the liver largely occurs after birth. In the early stages of life, the liver of a newborn encounters enormous high-fat metabolic stress caused by the consumption of breast milk. It is unclear how the maturing liver adapts to high lipid metabolism. Liver sinusoidal endothelial cells (LSECs) play a fundamental role in establishing liver vasculature and are decorated with many glycoproteins on their surface. The gene encodes a cytidine-5'-monophosphate (CMP)-sialic acid transporter responsible for transporting CMP-sialic acids between the cytoplasm and the Golgi apparatus for protein sialylation. This study aimed to determine whether endothelial sialylation plays a role in hepatic vasculogenesis and functional maturation. Endothelial-specific knockout mice were generated. Liver tissues were collected for histologic analysis, lipidomic profiling, RNA sequencing, confocal immunofluorescence, and immunoblot analyses. Endothelial -deficient mice exhibited excessive neonatal hepatic lipid deposition, severe liver damage, and high mortality. Endothelial deletion of led to sinusoidal capillarization and disrupted hepatic zonation. Mechanistically, vascular endothelial growth factor receptor 2 (VEGFR2) in LSECs was desialylated and VEGFR2 signaling was enhanced in -deficient mice. Inhibition of VEGFR2 signaling by SU5416 alleviated lipid deposition and restored hepatic vasculature in -deficient mice. Our findings suggest that sialylation of LSECs is critical for maintaining hepatic vascular development and lipid homeostasis. Targeting VEGFR2 signaling may be a new strategy to prevent liver disorders associated with abnormal vasculature and lipid deposition.
中文翻译:

内皮 Slc35a1 缺陷导致 LSEC 特性丧失并加剧小鼠肝脏中的新生脂质沉积
肝脏的功能成熟主要发生在出生后。在生命的早期阶段,新生儿的肝脏会因母乳的消耗而承受巨大的高脂肪代谢压力。目前尚不清楚成熟的肝脏如何适应高脂质代谢。肝窦内皮细胞(LSEC)在建立肝脏血管系统中发挥着重要作用,并且其表面装饰有许多糖蛋白。该基因编码胞苷-5'-单磷酸 (CMP)-唾液酸转运蛋白,负责在细胞质和高尔基体之间转运 CMP-唾液酸以进行蛋白质唾液酸化。本研究旨在确定内皮唾液酸化是否在肝血管生成和功能成熟中发挥作用。产生了内皮特异性基因敲除小鼠。收集肝组织用于组织学分析、脂质组学分析、RNA测序、共聚焦免疫荧光和免疫印迹分析。内皮缺陷小鼠表现出过度的新生肝脏脂质沉积、严重的肝损伤和高死亡率。内皮缺失导致肝窦毛细血管化并破坏肝分区。从机制上讲,LSEC 中的血管内皮生长因子受体 2 (VEGFR2) 被去唾液酸化,并且 VEGFR2 信号传导在缺陷小鼠中得到增强。 SU5416 对 VEGFR2 信号传导的抑制可减轻脂质沉积并恢复缺陷小鼠的肝血管系统。我们的研究结果表明,LSEC 的唾液酸化对于维持肝血管发育和脂质稳态至关重要。靶向 VEGFR2 信号传导可能是预防与异常脉管系统和脂质沉积相关的肝脏疾病的新策略。
更新日期:2024-03-11
中文翻译:

内皮 Slc35a1 缺陷导致 LSEC 特性丧失并加剧小鼠肝脏中的新生脂质沉积
肝脏的功能成熟主要发生在出生后。在生命的早期阶段,新生儿的肝脏会因母乳的消耗而承受巨大的高脂肪代谢压力。目前尚不清楚成熟的肝脏如何适应高脂质代谢。肝窦内皮细胞(LSEC)在建立肝脏血管系统中发挥着重要作用,并且其表面装饰有许多糖蛋白。该基因编码胞苷-5'-单磷酸 (CMP)-唾液酸转运蛋白,负责在细胞质和高尔基体之间转运 CMP-唾液酸以进行蛋白质唾液酸化。本研究旨在确定内皮唾液酸化是否在肝血管生成和功能成熟中发挥作用。产生了内皮特异性基因敲除小鼠。收集肝组织用于组织学分析、脂质组学分析、RNA测序、共聚焦免疫荧光和免疫印迹分析。内皮缺陷小鼠表现出过度的新生肝脏脂质沉积、严重的肝损伤和高死亡率。内皮缺失导致肝窦毛细血管化并破坏肝分区。从机制上讲,LSEC 中的血管内皮生长因子受体 2 (VEGFR2) 被去唾液酸化,并且 VEGFR2 信号传导在缺陷小鼠中得到增强。 SU5416 对 VEGFR2 信号传导的抑制可减轻脂质沉积并恢复缺陷小鼠的肝血管系统。我们的研究结果表明,LSEC 的唾液酸化对于维持肝血管发育和脂质稳态至关重要。靶向 VEGFR2 信号传导可能是预防与异常脉管系统和脂质沉积相关的肝脏疾病的新策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号