当前位置:
X-MOL 学术
›
Carbohydr. Polym.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Protein-assisted synthesis of chitosan-coated minicells enhance dendritic cell recruitment for therapeutic immunomodulation within pulmonary tumors
Carbohydrate Polymers ( IF 11.2 ) Pub Date : 2024-03-08 , DOI: 10.1016/j.carbpol.2024.122031 Jing Feng , Yiting Liu , Xiaoran Zheng , Min Gao , Li Wang , Lígia R. Rodrigues , Yuting Wen , Hangcheng Pan , Gege Li , Longjiang Zhang , Bing Wan , Yunlei Zhang
Carbohydrate Polymers ( IF 11.2 ) Pub Date : 2024-03-08 , DOI: 10.1016/j.carbpol.2024.122031 Jing Feng , Yiting Liu , Xiaoran Zheng , Min Gao , Li Wang , Lígia R. Rodrigues , Yuting Wen , Hangcheng Pan , Gege Li , Longjiang Zhang , Bing Wan , Yunlei Zhang
|
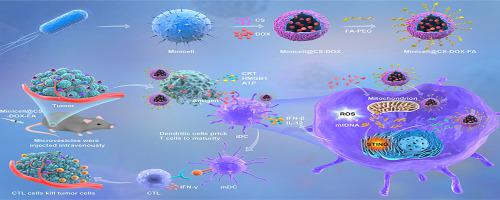
The efficacy of cancer therapies is significantly compromised by the immunosuppressive tumor milieu. Herein, we introduce a previously unidentified therapeutic strategy that harnesses the synergistic potential of chitosan-coated bacterial vesicles and a targeted chemotherapeutic agent to activate dendritic cells, thereby reshaping the immunosuppressive milieu for enhanced cancer therapy. Our study focuses on the protein-mediated modification of bacterium-derived minicells with chitosan molecules, facilitating the precise delivery of Doxorubicin to tumor sites guided by folate-mediated homing cues. These engineered minicells demonstrate remarkable specificity in targeting lung carcinomas, triggering immunogenic cell death and releasing tumor antigens and damage-associated molecular patterns, including calreticulin and high mobility group box 1. Additionally, the chitosan coating, coupled with bacterial DNA from the minicells, initiates the generation of reactive oxygen species and mitochondrial DNA release. These orchestrated events culminate in dendritic cell maturation activation of the stimulator of interferon genes signaling pathway, resulting in the recruitment of CD4 and CD8 cytotoxic T cells and the secretion of interferon-β, interferon-γ, and interleukin-12. Consequently, this integrated approach disrupts the immunosuppressive tumor microenvironment, impeding tumor progression. By leveraging bacterial vesicles as potent dendritic cell activators, our strategy presents a promising paradigm for synergistic cancer treatment, seamlessly integrating chemotherapy and immunotherapy.
中文翻译:

蛋白质辅助合成壳聚糖包被的小细胞增强树突状细胞募集,用于肺部肿瘤内的治疗性免疫调节
免疫抑制肿瘤环境显着损害了癌症治疗的功效。在此,我们介绍了一种先前未确定的治疗策略,该策略利用壳聚糖包被的细菌囊泡和靶向化疗剂的协同潜力来激活树突状细胞,从而重塑免疫抑制环境以增强癌症治疗。我们的研究重点是用壳聚糖分子对细菌来源的小细胞进行蛋白质介导的修饰,促进阿霉素在叶酸介导的归巢信号引导下精确递送至肿瘤部位。这些工程微细胞在靶向肺癌方面表现出显着的特异性,触发免疫原性细胞死亡并释放肿瘤抗原和损伤相关分子模式,包括钙网蛋白和高迁移率族盒1。此外,壳聚糖涂层与来自微细胞的细菌DNA结合,启动活性氧的产生和线粒体 DNA 的释放。这些精心策划的事件最终导致干扰素基因信号通路刺激剂的树突状细胞成熟激活,导致 CD4 和 CD8 细胞毒性 T 细胞的募集以及干扰素-β、干扰素-γ 和白介素-12 的分泌。因此,这种综合方法破坏了免疫抑制性肿瘤微环境,阻碍了肿瘤进展。通过利用细菌囊泡作为有效的树突状细胞激活剂,我们的策略为协同癌症治疗提供了一个有前途的范例,无缝整合化疗和免疫疗法。
更新日期:2024-03-08
中文翻译:

蛋白质辅助合成壳聚糖包被的小细胞增强树突状细胞募集,用于肺部肿瘤内的治疗性免疫调节
免疫抑制肿瘤环境显着损害了癌症治疗的功效。在此,我们介绍了一种先前未确定的治疗策略,该策略利用壳聚糖包被的细菌囊泡和靶向化疗剂的协同潜力来激活树突状细胞,从而重塑免疫抑制环境以增强癌症治疗。我们的研究重点是用壳聚糖分子对细菌来源的小细胞进行蛋白质介导的修饰,促进阿霉素在叶酸介导的归巢信号引导下精确递送至肿瘤部位。这些工程微细胞在靶向肺癌方面表现出显着的特异性,触发免疫原性细胞死亡并释放肿瘤抗原和损伤相关分子模式,包括钙网蛋白和高迁移率族盒1。此外,壳聚糖涂层与来自微细胞的细菌DNA结合,启动活性氧的产生和线粒体 DNA 的释放。这些精心策划的事件最终导致干扰素基因信号通路刺激剂的树突状细胞成熟激活,导致 CD4 和 CD8 细胞毒性 T 细胞的募集以及干扰素-β、干扰素-γ 和白介素-12 的分泌。因此,这种综合方法破坏了免疫抑制性肿瘤微环境,阻碍了肿瘤进展。通过利用细菌囊泡作为有效的树突状细胞激活剂,我们的策略为协同癌症治疗提供了一个有前途的范例,无缝整合化疗和免疫疗法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号