当前位置:
X-MOL 学术
›
Carbohydr. Polym.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of β-cyclodextrin derivatives substituted at larger or smaller rims via amine-catalyzed ring-opening oligomerization of ε-caprolactone
Carbohydrate Polymers ( IF 11.2 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.carbpol.2024.122032 Diana-Andreea Blaj , Mihaela Balan-Porcarasu , Valeria Harabagiu , Cristian Peptu
Carbohydrate Polymers ( IF 11.2 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.carbpol.2024.122032 Diana-Andreea Blaj , Mihaela Balan-Porcarasu , Valeria Harabagiu , Cristian Peptu
|
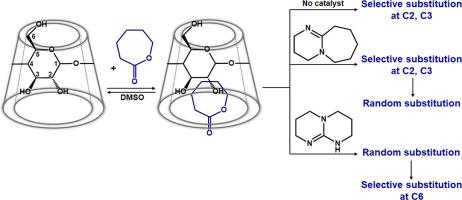
The involvement of cyclodextrins in transesterification reactions with active esters has been described to mimic enzyme-catalyzed reactions, making cyclodextrin molecules suitable as enzyme models. Cyclodextrin-catalyzed ring-opening of cyclic esters in bulk reaction conditions was considered to proceed similarly. However, the mechanism of activating cyclic esters through inclusion in the cyclodextrin cavity remains incompletely understood to date. The present research is focused on observing the transesterification of ε-caprolactone in the presence of β-cyclodextrin and additional amine organocatalysts within dimethyl sulfoxide solutions. The conducted experiments provide insights into the structural changes caused by various catalytic conditions in terms of the substitution pattern of the cyclodextrins. Our results are supported by a deep structural characterization through NMR and MALDI MS, which revealed the prospect of promoting rim-selective substitution of β-cyclodextrin at either secondary or primary hydroxyl groups using 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) organocatalysts. This offers the possibility to prepare cyclodextrin derivatives with specific substitution patterns. Based on the acquired structural information, the particular pathway in which β-cyclodextrin influences the ring-opening of ε-caprolactone is delineated as follows: monomer complexation, substitution at the larger rim, chain elongation, and intramolecular transfer toward the smaller rim.
中文翻译:

通过胺催化ε-己内酯的开环低聚合成在较大或较小边缘取代的β-环糊精衍生物
环糊精参与与活性酯的酯交换反应已被描述为模拟酶催化反应,使得环糊精分子适合作为酶模型。在本体反应条件下,环糊精催化的环酯开环被认为以类似方式进行。然而,迄今为止,通过包含在环糊精空腔中来激活环酯的机制仍不完全清楚。目前的研究重点是观察二甲亚砜溶液中 β-环糊精和其他胺有机催化剂存在下 ε-己内酯的酯交换反应。所进行的实验提供了对环糊精取代模式方面由各种催化条件引起的结构变化的见解。我们的结果得到了通过 NMR 和 MALDI MS 进行的深入结构表征的支持,这揭示了使用 1,8-二氮杂双环[5.4.0]undec-7 在仲羟基或伯羟基上促进 β-环糊精的边缘选择性取代的前景-ene (DBU) 和 1,5,7-三氮杂双环[4.4.0]dec-5-ene (TBD) 有机催化剂。这提供了制备具有特定取代模式的环糊精衍生物的可能性。根据获得的结构信息,描述了β-环糊精影响ε-己内酯开环的具体途径如下:单体络合、大环取代、链伸长和向小环分子内转移。
更新日期:2024-03-07
中文翻译:

通过胺催化ε-己内酯的开环低聚合成在较大或较小边缘取代的β-环糊精衍生物
环糊精参与与活性酯的酯交换反应已被描述为模拟酶催化反应,使得环糊精分子适合作为酶模型。在本体反应条件下,环糊精催化的环酯开环被认为以类似方式进行。然而,迄今为止,通过包含在环糊精空腔中来激活环酯的机制仍不完全清楚。目前的研究重点是观察二甲亚砜溶液中 β-环糊精和其他胺有机催化剂存在下 ε-己内酯的酯交换反应。所进行的实验提供了对环糊精取代模式方面由各种催化条件引起的结构变化的见解。我们的结果得到了通过 NMR 和 MALDI MS 进行的深入结构表征的支持,这揭示了使用 1,8-二氮杂双环[5.4.0]undec-7 在仲羟基或伯羟基上促进 β-环糊精的边缘选择性取代的前景-ene (DBU) 和 1,5,7-三氮杂双环[4.4.0]dec-5-ene (TBD) 有机催化剂。这提供了制备具有特定取代模式的环糊精衍生物的可能性。根据获得的结构信息,描述了β-环糊精影响ε-己内酯开环的具体途径如下:单体络合、大环取代、链伸长和向小环分子内转移。



























 京公网安备 11010802027423号
京公网安备 11010802027423号