当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Statistical analysis of sequential motifs at biologically relevant protein-protein interfaces
Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.csbj.2024.03.004 Yair Frank , Ron Unger , Hanoch Senderowitz
Computational and Structural Biotechnology Journal ( IF 6 ) Pub Date : 2024-03-07 , DOI: 10.1016/j.csbj.2024.03.004 Yair Frank , Ron Unger , Hanoch Senderowitz
|
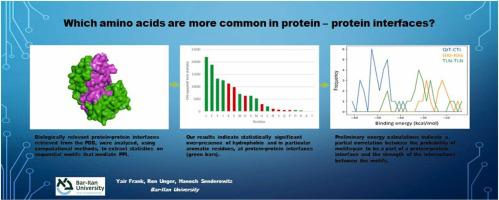
Understanding protein-protein interactions (PPIs) at the molecular level may lead to innovations in medicine and biochemistry. The assumption that there are certain “hot spots” on protein surfaces that mediate their interactions with other proteins has led to a search for specific sequences involved in protein-protein contacts. In this work, we analyze sequential amino acid motifs, both at the single motif and at the motif-motif level, across a large and diverse dataset of biologically relevant protein-protein interfaces retrieved from the PDB, comparing their presence at interfaces and surfaces in a statistically rigorous manner. At the single motif level, our results indicate statistically significant over-presence of hydrophobic and in particular aromatic residues and under-presence of charged residues at protein-protein interfaces. Certain PPI-mediating motifs reported in the literature (, the Tyrosine-based Motif YxxΦ and the PDZ-Binding Motif X-S/T-X-V/I) were confirmed to have a significant presence at interfaces. In addition, multiple PPI-mediating motifs were reported in the ELM database and from those present in our dataset, half were confirmed to have a statistically significant presence at interfaces whereas others were not. At the single residue, motif-motif level, Cysteine-Cysteine contacts were found to be the most abundant ones followed by interactions involving aromatic/hydrophobic residues. Top ranking, longer motif-motif pairs show predominance of Leucine and aromatic residues. Finally, preliminary energy calculations (using the MM/GBSA procedure) indicate a partial correlation between the probability of motifs-pair to be a part of a protein-protein interface and the strength of the interactions between the motifs. In conclusion, this study points to specific characteristics of motifs that have a higher probability to mediate protein-protein interactions. Prominent motifs identified in this study may be used in the future as possible components in protein engineering.
中文翻译:

生物学相关蛋白质-蛋白质界面的连续基序的统计分析
在分子水平上了解蛋白质-蛋白质相互作用(PPI)可能会带来医学和生物化学领域的创新。蛋白质表面上存在某些介导其与其他蛋白质相互作用的“热点”的假设导致人们寻找涉及蛋白质-蛋白质接触的特定序列。在这项工作中,我们分析了从 PDB 检索到的生物相关蛋白质-蛋白质界面的大型且多样化的数据集,在单个基序和基序-基序水平上分析了顺序氨基酸基序,比较了它们在界面和表面上的存在情况。统计上严格的方式。在单基序水平上,我们的结果表明,在蛋白质-蛋白质界面处,疏水性残基(特别是芳香族残基)的存在具有统计学显着性,而带电残基的存在性不足。文献中报道的某些 PPI 介导基序(基于酪氨酸的基序 YxxΦ 和 PDZ 结合基序 XS/TXV/I)被证实在界面处显着存在。此外,ELM 数据库中报告了多个 PPI 介导基序,并且从我们数据集中存在的基序来看,其中一半被确认在界面处具有统计显着性,而其他基序则没有。在单个残基、基序水平上,发现半胱氨酸-半胱氨酸接触是最丰富的,其次是涉及芳香族/疏水性残基的相互作用。排名靠前、较长的基序对显示亮氨酸和芳香族残基占主导地位。最后,初步能量计算(使用 MM/GBSA 程序)表明基序对成为蛋白质-蛋白质界面一部分的概率与基序之间相互作用的强度之间存在部分相关性。总之,这项研究指出了更有可能介导蛋白质-蛋白质相互作用的基序的特定特征。本研究中确定的突出基序可能在未来用作蛋白质工程的可能组成部分。
更新日期:2024-03-07
中文翻译:

生物学相关蛋白质-蛋白质界面的连续基序的统计分析
在分子水平上了解蛋白质-蛋白质相互作用(PPI)可能会带来医学和生物化学领域的创新。蛋白质表面上存在某些介导其与其他蛋白质相互作用的“热点”的假设导致人们寻找涉及蛋白质-蛋白质接触的特定序列。在这项工作中,我们分析了从 PDB 检索到的生物相关蛋白质-蛋白质界面的大型且多样化的数据集,在单个基序和基序-基序水平上分析了顺序氨基酸基序,比较了它们在界面和表面上的存在情况。统计上严格的方式。在单基序水平上,我们的结果表明,在蛋白质-蛋白质界面处,疏水性残基(特别是芳香族残基)的存在具有统计学显着性,而带电残基的存在性不足。文献中报道的某些 PPI 介导基序(基于酪氨酸的基序 YxxΦ 和 PDZ 结合基序 XS/TXV/I)被证实在界面处显着存在。此外,ELM 数据库中报告了多个 PPI 介导基序,并且从我们数据集中存在的基序来看,其中一半被确认在界面处具有统计显着性,而其他基序则没有。在单个残基、基序水平上,发现半胱氨酸-半胱氨酸接触是最丰富的,其次是涉及芳香族/疏水性残基的相互作用。排名靠前、较长的基序对显示亮氨酸和芳香族残基占主导地位。最后,初步能量计算(使用 MM/GBSA 程序)表明基序对成为蛋白质-蛋白质界面一部分的概率与基序之间相互作用的强度之间存在部分相关性。总之,这项研究指出了更有可能介导蛋白质-蛋白质相互作用的基序的特定特征。本研究中确定的突出基序可能在未来用作蛋白质工程的可能组成部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号