当前位置:
X-MOL 学术
›
Carbon Energy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effect of salt anion in ether-based electrolyte for electrochemical performance of sodium-ion batteries: A case study of hard carbon
Carbon Energy ( IF 20.5 ) Pub Date : 2024-03-13 , DOI: 10.1002/cey2.518 Jiabao Li 1 , Jingjing Hao 1 , Quan Yuan 1 , Ruoxing Wang 1 , Frederick Marlton 2 , Tianyi Wang 1 , Chengyin Wang 1 , Xin Guo 2 , Guoxiu Wang 2
Carbon Energy ( IF 20.5 ) Pub Date : 2024-03-13 , DOI: 10.1002/cey2.518 Jiabao Li 1 , Jingjing Hao 1 , Quan Yuan 1 , Ruoxing Wang 1 , Frederick Marlton 2 , Tianyi Wang 1 , Chengyin Wang 1 , Xin Guo 2 , Guoxiu Wang 2
Affiliation
|
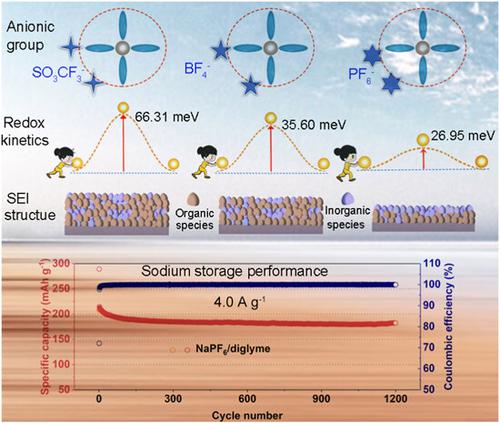
Compared with the extensively used ester-based electrolyte, the hard carbon (HC) electrode is more compatible with the ether-based counterpart in sodium-ion batteries, which can lead to improved cycling stability and robust rate capability. However, the impact of salt anion on the electrochemical performance of HC electrodes has yet to be fully understood. In this study, the anionic chemistry in regulating the stability of electrolytes and the performance of sodium-ion batteries have been systematically investigated. This work shows discrepancies in the reductive stability of the anionic group, redox kinetics, and component/structure of solid electrolyte interface (SEI) with different salts (NaBF4, NaPF6, and NaSO3CF3) in the typical ether solvent (diglyme). Particularly, the density functional theory calculation manifests the preferred decomposition of PF6− due to the reduced reductive stability of anions in the solvation structure, thus leading to the formation of NaF-rich SEI. Further investigation on redox kinetics reveals that the NaPF6/diglyme can induce the fast ionic diffusion dynamic and low charge transfer barrier for HC electrode, thus resulting in superior sodium storage performance in terms of rate capability and cycling life, which outperforms those of NaBF4/diglyme and NaSO3CF3/diglyme. Importantly, this work offers valuable insights for optimizing the electrochemical behaviors of electrode materials by regulating the anionic group in the electrolyte.
中文翻译:

醚基电解液中盐阴离子对钠离子电池电化学性能的影响:以硬碳为例
与广泛使用的酯基电解质相比,硬碳(HC)电极与钠离子电池中的醚基电解质更相容,这可以提高循环稳定性和鲁棒的倍率性能。然而,盐阴离子对HC电极电化学性能的影响尚未完全了解。在这项研究中,系统地研究了调节电解质稳定性和钠离子电池性能的阴离子化学。这项工作表明,在典型的醚溶剂(二甘醇二甲醚)中,不同盐(NaBF 4 、 NaPF 6和NaSO 3 CF 3)的阴离子基团的还原稳定性、氧化还原动力学和固体电解质界面(SEI)的成分/结构存在差异。 )。特别是,密度泛函理论计算表明,由于溶剂化结构中阴离子的还原稳定性降低,PF 6 -优先分解,从而导致富含NaF的SEI的形成。氧化还原动力学的进一步研究表明,NaPF 6 /二甘醇二甲醚可以诱导HC电极快速的离子扩散动力学和低的电荷转移势垒,从而在倍率性能和循环寿命方面产生优异的钠存储性能,优于NaBF 4 /二甘醇二甲醚和 NaSO 3 CF 3 /二甘醇二甲醚。重要的是,这项工作为通过调节电解质中的阴离子基团来优化电极材料的电化学行为提供了有价值的见解。
更新日期:2024-03-14
中文翻译:

醚基电解液中盐阴离子对钠离子电池电化学性能的影响:以硬碳为例
与广泛使用的酯基电解质相比,硬碳(HC)电极与钠离子电池中的醚基电解质更相容,这可以提高循环稳定性和鲁棒的倍率性能。然而,盐阴离子对HC电极电化学性能的影响尚未完全了解。在这项研究中,系统地研究了调节电解质稳定性和钠离子电池性能的阴离子化学。这项工作表明,在典型的醚溶剂(二甘醇二甲醚)中,不同盐(NaBF 4 、 NaPF 6和NaSO 3 CF 3)的阴离子基团的还原稳定性、氧化还原动力学和固体电解质界面(SEI)的成分/结构存在差异。 )。特别是,密度泛函理论计算表明,由于溶剂化结构中阴离子的还原稳定性降低,PF 6 -优先分解,从而导致富含NaF的SEI的形成。氧化还原动力学的进一步研究表明,NaPF 6 /二甘醇二甲醚可以诱导HC电极快速的离子扩散动力学和低的电荷转移势垒,从而在倍率性能和循环寿命方面产生优异的钠存储性能,优于NaBF 4 /二甘醇二甲醚和 NaSO 3 CF 3 /二甘醇二甲醚。重要的是,这项工作为通过调节电解质中的阴离子基团来优化电极材料的电化学行为提供了有价值的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号