当前位置:
X-MOL 学术
›
Cancer Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
KLK10 derived from tumor endothelial cells accelerates colon cancer cell proliferation and hematogenous liver metastasis formation
Cancer Science ( IF 5.7 ) Pub Date : 2024-03-13 , DOI: 10.1111/cas.16144 Kazuya Kato 1 , Takehiro Noda 1 , Shogo Kobayashi 1 , Kazuki Sasaki 1 , Yoshifumi Iwagami 1 , Daisaku Yamada 1 , Yoshito Tomimaru 1 , Hidenori Takahashi 1 , Mamoru Uemura 1 , Tadafumi Asaoka 1 , Junzo Shimizu 1 , Yuichiro Doki 1 , Hidetoshi Eguchi 1
Cancer Science ( IF 5.7 ) Pub Date : 2024-03-13 , DOI: 10.1111/cas.16144 Kazuya Kato 1 , Takehiro Noda 1 , Shogo Kobayashi 1 , Kazuki Sasaki 1 , Yoshifumi Iwagami 1 , Daisaku Yamada 1 , Yoshito Tomimaru 1 , Hidenori Takahashi 1 , Mamoru Uemura 1 , Tadafumi Asaoka 1 , Junzo Shimizu 1 , Yuichiro Doki 1 , Hidetoshi Eguchi 1
Affiliation
|
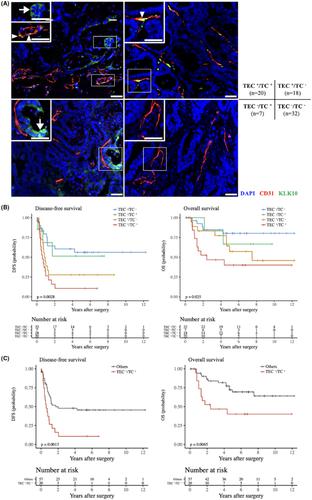
Tumor endothelial cells (TECs), which are thought to be structurally and functionally different from normal endothelial cells (NECs), are increasingly attracting attention as a therapeutic target in hypervascular malignancies. Although colorectal liver metastasis (CRLM) tumors are hypovascular, inhibitors of angiogenesis are a key drug in multidisciplinary therapy, and TECs might be involved in the development and progression of cancer. Here, we analyzed the function of TEC in the CRLM tumor microenvironment. We used a murine colon cancer cell line (CT26) and isolated TECs from CRLM tumors. TECs showed higher proliferation and migration than NECs. Coinjection of CT26 and TECs yielded rapid tumor formation in vivo. Immunofluorescence analysis showed that coinjection of CT26 and TECs increased vessel formation and Ki‐67+ cells. Transcriptome analysis identified kallikrein‐related peptide 10 (KLK10) as a candidate target. Coinjection of CT26 and TECs after KLK10 downregulation with siRNA suppressed tumor formation in vivo. TEC secretion of KLK10 decreased after KLK10 downregulation, and conditioned medium after KLK10 knockdown in TECs suppressed CT26 proliferative activity. Double immunofluorescence staining of KLK10 and CD31 in CRLM tissues revealed a significant correlation between poor prognosis and positive KLK10 expression in TECs and tumor cells. On multivariate analysis, KLK10 expression was an independent prognostic factor in disease‐free survival. In conclusion, KLK10 derived from TECs accelerates colon cancer cell proliferation and hematogenous liver metastasis formation. KLK10 in TECs might offer a promising therapeutic target in CRLM.
中文翻译:

肿瘤内皮细胞来源的KLK10加速结肠癌细胞增殖和血行肝转移形成
肿瘤内皮细胞(TEC)被认为在结构和功能上与正常内皮细胞(NEC)不同,作为富血管恶性肿瘤的治疗靶点越来越受到关注。尽管结直肠肝转移 (CRLM) 肿瘤缺乏血管,但血管生成抑制剂是多学科治疗中的关键药物,并且 TEC 可能参与癌症的发生和进展。在这里,我们分析了 TEC 在 CRLM 肿瘤微环境中的功能。我们使用小鼠结肠癌细胞系 (CT26) 并从 CRLM 肿瘤中分离出 TEC。TECs 表现出比 NECs 更高的增殖和迁移能力。CT26 和 TEC 的共注射导致体内肿瘤快速形成。免疫荧光分析表明,CT26 和 TEC 的共注射增加了血管形成和 Ki-67+ 细胞。转录组分析将激肽释放酶相关肽 10 (KLK10) 确定为候选靶点。KLK10 与 siRNA 下调后共注射 CT26 和 TEC 可抑制体内肿瘤形成。KLK10下调后,KLK10的TEC分泌减少,TEC中KLK10敲低后的条件培养基抑制了CT26增殖活性。CRLM 组织中 KLK10 和 CD31 的双重免疫荧光染色揭示不良预后与 TEC 和肿瘤细胞中 KLK10 阳性表达之间存在显着相关性。多变量分析显示,KLK10 表达是无病生存的独立预后因素。总之,源自TEC的KLK10加速结肠癌细胞增殖和血行肝转移形成。TEC 中的 KLK10 可能为 CRLM 提供一个有前景的治疗靶点。
更新日期:2024-03-13
中文翻译:

肿瘤内皮细胞来源的KLK10加速结肠癌细胞增殖和血行肝转移形成
肿瘤内皮细胞(TEC)被认为在结构和功能上与正常内皮细胞(NEC)不同,作为富血管恶性肿瘤的治疗靶点越来越受到关注。尽管结直肠肝转移 (CRLM) 肿瘤缺乏血管,但血管生成抑制剂是多学科治疗中的关键药物,并且 TEC 可能参与癌症的发生和进展。在这里,我们分析了 TEC 在 CRLM 肿瘤微环境中的功能。我们使用小鼠结肠癌细胞系 (CT26) 并从 CRLM 肿瘤中分离出 TEC。TECs 表现出比 NECs 更高的增殖和迁移能力。CT26 和 TEC 的共注射导致体内肿瘤快速形成。免疫荧光分析表明,CT26 和 TEC 的共注射增加了血管形成和 Ki-67



























 京公网安备 11010802027423号
京公网安备 11010802027423号