当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Strengthening of Noncovalent Bonds Caused by Internal Deformations
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jpca.4c00541 Steve Scheiner 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jpca.4c00541 Steve Scheiner 1
Affiliation
|
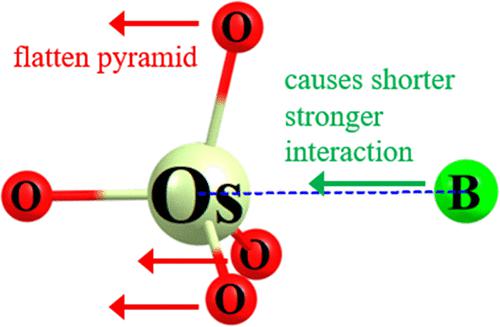
It is usually assumed that the maximal noncovalent bond strength is achieved by full geometry optimization of the geometry of the dyad. Density functional theory calculations show this not to be the case. A number of systems are considered that include osme, tetrel, pnictogen, and chalcogen bonds, involving both σ- and π-holes, as well as hypervalency. By suitable adjustment of the bond angles within the Lewis acid, the base can be drawn closer than in the optimized structure, with an accompanying substantial strengthening of the noncovalent bond, by more than 10 kcal/mol in some cases. The energetic cost of this deformation from the optimized geometry can be surprisingly small in comparison to the gain in the interaction energy. Taking the opposite approach of first pushing the two subunits closer to one another and then permitting internal geometries to adjust to the shortened distance produces only minimal bond strengthening.
中文翻译:

内部变形引起的非共价键强化
通常假设最大非共价键强度是通过对二元组的几何形状进行全面几何优化来实现的。密度泛函理论计算表明情况并非如此。许多系统被认为包括 osme、tetrel、pnictogen 和 chalcogen 键,涉及 σ 和 π 空穴以及超价。通过适当调整路易斯酸内的键角,可以比优化结构中的碱基更接近,同时伴随着非共价键的显着增强,在某些情况下增强超过 10 kcal/mol。与相互作用能的增益相比,优化几何形状产生的这种变形的能量成本可能小得惊人。采用相反的方法,首先将两个亚基彼此靠近,然后允许内部几何形状调整以适应缩短的距离,仅产生最小的键合强化。
更新日期:2024-03-14
中文翻译:

内部变形引起的非共价键强化
通常假设最大非共价键强度是通过对二元组的几何形状进行全面几何优化来实现的。密度泛函理论计算表明情况并非如此。许多系统被认为包括 osme、tetrel、pnictogen 和 chalcogen 键,涉及 σ 和 π 空穴以及超价。通过适当调整路易斯酸内的键角,可以比优化结构中的碱基更接近,同时伴随着非共价键的显着增强,在某些情况下增强超过 10 kcal/mol。与相互作用能的增益相比,优化几何形状产生的这种变形的能量成本可能小得惊人。采用相反的方法,首先将两个亚基彼此靠近,然后允许内部几何形状调整以适应缩短的距离,仅产生最小的键合强化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号