当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Consecutive Reduction of Five Carbon Dioxide Molecules by Gas-Phase Niobium Carbide Cluster Anions Nb3C4–: Unusual Mechanism for Enhanced Reactivity by the Carbon Ligands
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jpca.4c00371 Yi-Heng Zhang 1 , Jia-Bi Ma 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-14 , DOI: 10.1021/acs.jpca.4c00371 Yi-Heng Zhang 1 , Jia-Bi Ma 1
Affiliation
|
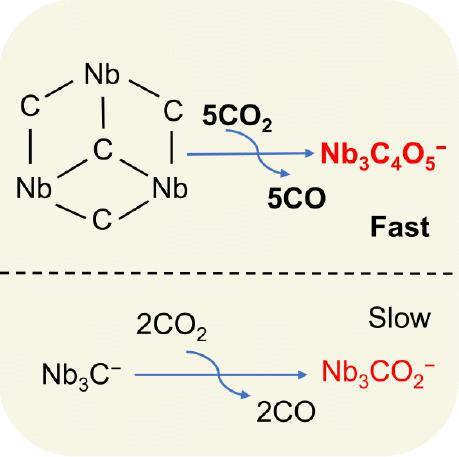
Studying the cleavage of the C═O bond during CO2 activation at room temperature is highly significant for comprehending the CO2 conversion processes. Herein, mass spectrometry experiments and density functional theory calculations indicate that the niobium carbide anions Nb3C4– can continuously convert five CO2 molecules to CO under thermal collision conditions, while the other clusters with less carbon ligands Nb3C1–3– reduce fewer CO2 molecules. Size-dependent reactivity of Nb3C1–4– cluster anions toward CO2 is observed. Interestingly, the carbon atoms in Nb3C4– not only act as highly active adsorption sites for CO2 but also serve as electron donors to reduce CO2. The stored electrons are released through a carbon–carbon coupling process. Our findings on the role of carbon ligands in enhancing transition metal carbide reactivity can offer new insights for designing active sites on catalysts with both high activity and selectivity.
中文翻译:

气相碳化铌簇阴离子 Nb3C4– 连续还原五个二氧化碳分子:碳配体增强反应性的不寻常机制
研究室温下CO 2活化过程中C=O键的断裂对于理解CO 2转化过程具有重要意义。其中,质谱实验和密度泛函理论计算表明,碳化铌阴离子Nb 3 C 4 –在热碰撞条件下可以连续将5个CO 2分子转化为CO,而其他碳配体较少的团簇Nb 3 C 1–3 –减少更少的CO 2分子。观察到Nb 3 C 1–4 -簇阴离子对 CO 2的尺寸依赖性反应性。有趣的是,Nb 3 C 4中的碳原子不仅充当CO 2的高活性吸附位点,而且还充当还原CO 2的电子供体。储存的电子通过碳-碳耦合过程释放。我们关于碳配体在增强过渡金属碳化物反应活性方面的作用的发现可以为设计具有高活性和选择性的催化剂活性位点提供新的见解。
更新日期:2024-03-14
中文翻译:

气相碳化铌簇阴离子 Nb3C4– 连续还原五个二氧化碳分子:碳配体增强反应性的不寻常机制
研究室温下CO 2活化过程中C=O键的断裂对于理解CO 2转化过程具有重要意义。其中,质谱实验和密度泛函理论计算表明,碳化铌阴离子Nb 3 C 4 –在热碰撞条件下可以连续将5个CO 2分子转化为CO,而其他碳配体较少的团簇Nb 3 C 1–3 –减少更少的CO 2分子。观察到Nb 3 C 1–4 -簇阴离子对 CO 2的尺寸依赖性反应性。有趣的是,Nb 3 C 4中的碳原子不仅充当CO 2的高活性吸附位点,而且还充当还原CO 2的电子供体。储存的电子通过碳-碳耦合过程释放。我们关于碳配体在增强过渡金属碳化物反应活性方面的作用的发现可以为设计具有高活性和选择性的催化剂活性位点提供新的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号