当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Shapeshifting Nucleophile Singly Hydrated Hydroperoxide Anion Leads to the Occurrence of the Thermodynamically Unfavored SN2 Product
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-13 , DOI: 10.1021/acs.jpca.4c01159 Xiangyu Wu 1 , Chongyang Zhao 1 , Shaowen Zhang 1 , Jing Xie 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-13 , DOI: 10.1021/acs.jpca.4c01159 Xiangyu Wu 1 , Chongyang Zhao 1 , Shaowen Zhang 1 , Jing Xie 1
Affiliation
|
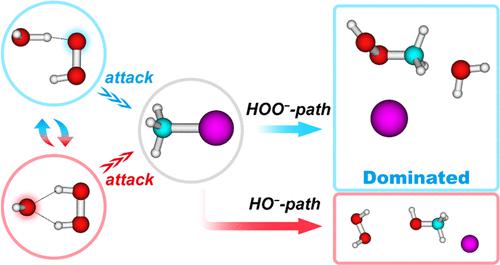
Single water molecules alone may introduce unusual features into the kinetics and dynamics of chemical reactions. The singly hydrated hydroperoxide anion, HOO–(H2O), was found to be a shapeshifting nucleophile, which can be transformed to HO– solvated by hydrogen peroxide HO–(HOOH). Herein, we performed direct dynamics simulations of its reaction with methyl iodide to investigate the effect of individual water molecules. In addition to the normal SN2 product CH3OOH, the thermodynamically unfavored proton transfer-induced HO–-SN2 path (produces CH3OH) was also observed, contributing ∼4%. The simulated branching ratio of the HO–-SN2 path exceeded the statistical estimation (0.6%) based on the free energy barrier difference. The occurrence of the HO–-SN2 path was attributed to the shallow entrance channel well before a submerged saddle point, thus providing a region for extensive proton exchange and ultimately leading to the formation of CH3OH. In comparison, changing the leaving group from Cl to I increased the overall reaction rate as well as the proportion of the HO–-SN2 path because the CH3I system has a smaller internal barrier. This work elucidates the importance of the dynamic effect introduced by a single solvent molecule to alter the product channel and kinetics of typical ion–molecule SN2 reactions.
中文翻译:

变形亲核试剂单水合氢过氧化物阴离子导致热力学不利的 SN2 产物的出现
单独的水分子可能会给化学反应的动力学和动力学带来不寻常的特征。单水合氢过氧化物阴离子 HOO – (H 2 O) 被发现是一种变形亲核试剂,可被过氧化氢 HO – (HOOH) 转化为 H2O –溶剂化物。在这里,我们对其与碘甲烷的反应进行了直接动力学模拟,以研究单个水分子的影响。除了正常的S N 2 产物CH 3 OOH之外,还观察到热力学不利的质子转移诱导的HO – -S N 2 路径(产生CH 3 OH),贡献约4%。 HO – -S N 2 路径的模拟分支比超出了基于自由能垒差异的统计估计(0.6%)。 HO – -S N 2 路径的出现归因于水下鞍点之前的浅入口通道,从而为广泛的质子交换提供了区域,并最终导致CH 3 OH的形成。相比之下,将离去基团从Cl改为I增加了总反应速率以及HO – -S N 2 路径的比例,因为CH 3 I系统具有较小的内势垒。这项工作阐明了单个溶剂分子引入的动态效应对于改变典型离子-分子 S N 2 反应的产物通道和动力学的重要性。
更新日期:2024-03-13
中文翻译:

变形亲核试剂单水合氢过氧化物阴离子导致热力学不利的 SN2 产物的出现
单独的水分子可能会给化学反应的动力学和动力学带来不寻常的特征。单水合氢过氧化物阴离子 HOO – (H 2 O) 被发现是一种变形亲核试剂,可被过氧化氢 HO – (HOOH) 转化为 H2O –溶剂化物。在这里,我们对其与碘甲烷的反应进行了直接动力学模拟,以研究单个水分子的影响。除了正常的S N 2 产物CH 3 OOH之外,还观察到热力学不利的质子转移诱导的HO – -S N 2 路径(产生CH 3 OH),贡献约4%。 HO – -S N 2 路径的模拟分支比超出了基于自由能垒差异的统计估计(0.6%)。 HO – -S N 2 路径的出现归因于水下鞍点之前的浅入口通道,从而为广泛的质子交换提供了区域,并最终导致CH 3 OH的形成。相比之下,将离去基团从Cl改为I增加了总反应速率以及HO – -S N 2 路径的比例,因为CH 3 I系统具有较小的内势垒。这项工作阐明了单个溶剂分子引入的动态效应对于改变典型离子-分子 S N 2 反应的产物通道和动力学的重要性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号