当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel chiral matrine derivatives as potential antitumor agents: Design, synthesis and biological evaluation
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-09 , DOI: 10.1016/j.bioorg.2024.107276 Gan Qiu , Fan Li , Jamal A.H. Kowah , Junwei Xie , Qingfeng Long , Lisheng Wang , Xu Liu
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-09 , DOI: 10.1016/j.bioorg.2024.107276 Gan Qiu , Fan Li , Jamal A.H. Kowah , Junwei Xie , Qingfeng Long , Lisheng Wang , Xu Liu
|
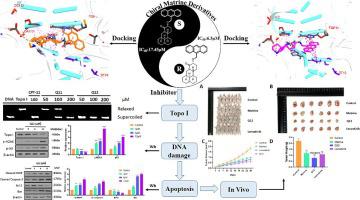
Since the thalidomide incident, research on chiral drugs has escalated immensely. Differences in drug configuration can lead to significant variations in therapeutic efficacy. Matrine, a natural product esteemed for its low toxicity and high water solubility, has garnered significant attention in research endeavors. Nonetheless, its precise target has proven elusive. In this study, we designed and synthesized a novel chiral matrine derivative. Their cytotoxicity against three types of tumor cells was assessed. Comparing the newly synthesized derivatives to the parent matrine, most compounds exhibited significantly enhanced inhibitory effects on cancer cells. Among them, exhibited the highest activity, with IC values of 8.31 μM against rat glioma cells C6, 6.3 μM against human liver cancer cells HepG2 and 7.14 μM against human gastric cancer cells HGC-27, meanwhile showing low toxicity. Based on IC values, we constructed a preliminary structure–activity relationship (SAR). Compound significantly suppressed the cloning and migration of HepG2 cells. Further mechanistic studies indicated that inhibited Topo I in HepG2 cells, leading to DNA damage, induction of G0/G1 cell cycle arrest and ultimately causing apoptosis. The molecular docking experiments provided a rational binding mode of with the Topo I-DNA complex. In vivo, experiments demonstrated that exhibited a higher tumor growth inhibition rate (TGI) compared to the positive control drug Lenvatinib, while maintaining good safety. In summary, it suggests that Topo I might be a potential target for matrine and represents a promising candidate for cancer treatment.
中文翻译:

作为潜在抗肿瘤药物的新型手性苦参碱衍生物:设计、合成和生物学评价
自沙利度胺事件以来,手性药物的研究急剧升级。药物配置的差异可能导致治疗效果的显着差异。苦参碱是一种天然产物,因其低毒性和高水溶性而受到人们的广泛关注。然而,事实证明其精确目标难以捉摸。在本研究中,我们设计并合成了一种新型手性苦参碱衍生物。评估了它们对三种类型肿瘤细胞的细胞毒性。将新合成的衍生物与母体苦参碱相比,大多数化合物对癌细胞的抑制作用显着增强。其中表现出最高的活性,对大鼠胶质瘤细胞C6的IC50值为8.31μM,对人肝癌细胞HepG2的IC50值为6.3μM,对人胃癌细胞HGC-27的IC50为7.14μM,同时表现出较低的毒性。根据IC值,我们构建了初步的结构-活性关系(SAR)。化合物显着抑制 HepG2 细胞的克隆和迁移。进一步的机制研究表明,抑制 HepG2 细胞中的 Topo I,导致 DNA 损伤,诱导 G0/G1 细胞周期停滞并最终导致细胞凋亡。分子对接实验提供了与Topo I-DNA复合物的合理结合模式。体内实验表明,与阳性对照药乐伐替尼相比,表现出更高的肿瘤生长抑制率(TGI),同时保持良好的安全性。总之,它表明 Topo I 可能是苦参碱的潜在靶点,并且是癌症治疗的有希望的候选者。
更新日期:2024-03-09
中文翻译:

作为潜在抗肿瘤药物的新型手性苦参碱衍生物:设计、合成和生物学评价
自沙利度胺事件以来,手性药物的研究急剧升级。药物配置的差异可能导致治疗效果的显着差异。苦参碱是一种天然产物,因其低毒性和高水溶性而受到人们的广泛关注。然而,事实证明其精确目标难以捉摸。在本研究中,我们设计并合成了一种新型手性苦参碱衍生物。评估了它们对三种类型肿瘤细胞的细胞毒性。将新合成的衍生物与母体苦参碱相比,大多数化合物对癌细胞的抑制作用显着增强。其中表现出最高的活性,对大鼠胶质瘤细胞C6的IC50值为8.31μM,对人肝癌细胞HepG2的IC50值为6.3μM,对人胃癌细胞HGC-27的IC50为7.14μM,同时表现出较低的毒性。根据IC值,我们构建了初步的结构-活性关系(SAR)。化合物显着抑制 HepG2 细胞的克隆和迁移。进一步的机制研究表明,抑制 HepG2 细胞中的 Topo I,导致 DNA 损伤,诱导 G0/G1 细胞周期停滞并最终导致细胞凋亡。分子对接实验提供了与Topo I-DNA复合物的合理结合模式。体内实验表明,与阳性对照药乐伐替尼相比,表现出更高的肿瘤生长抑制率(TGI),同时保持良好的安全性。总之,它表明 Topo I 可能是苦参碱的潜在靶点,并且是癌症治疗的有希望的候选者。



























 京公网安备 11010802027423号
京公网安备 11010802027423号