当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Qingkailing granule alleviates pulmonary fibrosis by inhibiting PI3K/AKT and SRC/STAT3 signaling pathways
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-13 , DOI: 10.1016/j.bioorg.2024.107286 Hong Li , Guang Xin , Qilong Zhou , Xiuxian Yu , Chengyu Wan , Yilan Wang , Ao Wen , Kun Zhang , Boli Zhang , Yu Cao , Wen Huang
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-13 , DOI: 10.1016/j.bioorg.2024.107286 Hong Li , Guang Xin , Qilong Zhou , Xiuxian Yu , Chengyu Wan , Yilan Wang , Ao Wen , Kun Zhang , Boli Zhang , Yu Cao , Wen Huang
|
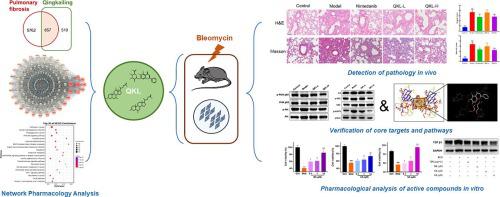
Pulmonary fibrosis (PF) poses a significant challenge with limited treatment options and a high mortality rate of approximately 45 %. Qingkailing Granule (QKL), derived from the Angong Niuhuang Pill, shows promise in addressing pulmonary conditions. Using a comprehensive approach, combining network pharmacology analysis with experimental validation, this study explores the therapeutic effects and mechanisms of QKL against PF for the first time. , QKL reduced collagen deposition and suppressed proinflammatory cytokines in a bleomycin-induced PF mouse model. studies demonstrated QKL's efficacy in protecting cells from bleomycin-induced injury and reducing collagen accumulation and cell migration in TGF-β1-induced pulmonary fibrosis cell models. Network pharmacology analysis revealed potential mechanisms, confirmed by western blotting, involving the modulation of PI3K/AKT and SRC/STAT3 signaling pathways. Molecular docking simulations highlighted interactions between QKL's active compounds and key proteins, showing inhibitory effects on epithelial damage and fibrosis. Collectively, these findings underscore the therapeutic potential of QKL in alleviating pulmonary inflammation and fibrosis through the downregulation of PI3K/AKT and SRC/STAT3 signaling pathways, with a pivotal role attributed to its active compounds.
中文翻译:

清开灵颗粒通过抑制PI3K/AKT和SRC/STAT3信号通路减轻肺纤维化
肺纤维化 (PF) 带来了重大挑战,治疗选择有限且死亡率高达约 45%。清开灵颗粒(QKL)源自安宫牛黄丸,在治疗肺部疾病方面显示出良好的前景。本研究采用综合方法,将网络药理学分析与实验验证相结合,首次探讨了 QKL 对 PF 的治疗作用和机制。在博来霉素诱导的 PF 小鼠模型中,QKL 减少了胶原蛋白沉积并抑制了促炎细胞因子。研究证明,在 TGF-β1 诱导的肺纤维化细胞模型中,QKL 可有效保护细胞免受博来霉素诱导的损伤,并减少胶原蛋白积累和细胞迁移。网络药理学分析揭示了涉及 PI3K/AKT 和 SRC/STAT3 信号通路调节的潜在机制,并经蛋白质印迹证实。分子对接模拟强调了 QKL 的活性化合物和关键蛋白质之间的相互作用,显示出对上皮损伤和纤维化的抑制作用。总的来说,这些发现强调了 QKL 通过下调 PI3K/AKT 和 SRC/STAT3 信号通路来减轻肺部炎症和纤维化的治疗潜力,其关键作用归因于其活性化合物。
更新日期:2024-03-13
中文翻译:

清开灵颗粒通过抑制PI3K/AKT和SRC/STAT3信号通路减轻肺纤维化
肺纤维化 (PF) 带来了重大挑战,治疗选择有限且死亡率高达约 45%。清开灵颗粒(QKL)源自安宫牛黄丸,在治疗肺部疾病方面显示出良好的前景。本研究采用综合方法,将网络药理学分析与实验验证相结合,首次探讨了 QKL 对 PF 的治疗作用和机制。在博来霉素诱导的 PF 小鼠模型中,QKL 减少了胶原蛋白沉积并抑制了促炎细胞因子。研究证明,在 TGF-β1 诱导的肺纤维化细胞模型中,QKL 可有效保护细胞免受博来霉素诱导的损伤,并减少胶原蛋白积累和细胞迁移。网络药理学分析揭示了涉及 PI3K/AKT 和 SRC/STAT3 信号通路调节的潜在机制,并经蛋白质印迹证实。分子对接模拟强调了 QKL 的活性化合物和关键蛋白质之间的相互作用,显示出对上皮损伤和纤维化的抑制作用。总的来说,这些发现强调了 QKL 通过下调 PI3K/AKT 和 SRC/STAT3 信号通路来减轻肺部炎症和纤维化的治疗潜力,其关键作用归因于其活性化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号