当前位置:
X-MOL 学术
›
Clin. Gastroenterol. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Long-Term Effectiveness and Durability of COVID-19 Vaccination Among Patients With Inflammatory Bowel Disease
Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2024-02-17 , DOI: 10.1016/j.cgh.2024.02.001 Erica J. Brenner , Kimberly N. Weaver , Xian Zhang , Arthur J. Kastl , Jennifer A. Strople , Jeremy Adler , Marla C. Dubinsky , Athos Bousvaros , Runa Watkins , Xiangfeng Dai , Wenli Chen , Raymond K. Cross , Peter D.R. Higgins , Ryan C. Ungaro , Meenakshi Bewtra , Emanuelle A. Bellaguarda , Francis A. Farraye , Kelly Y. Chun , Michael Zikry , Monique Bastidas , Ann Firestine , Riley G. Craig , Margie E. Boccieri , Millie D. Long , Michael D. Kappelman
Clinical Gastroenterology and Hepatology ( IF 12.6 ) Pub Date : 2024-02-17 , DOI: 10.1016/j.cgh.2024.02.001 Erica J. Brenner , Kimberly N. Weaver , Xian Zhang , Arthur J. Kastl , Jennifer A. Strople , Jeremy Adler , Marla C. Dubinsky , Athos Bousvaros , Runa Watkins , Xiangfeng Dai , Wenli Chen , Raymond K. Cross , Peter D.R. Higgins , Ryan C. Ungaro , Meenakshi Bewtra , Emanuelle A. Bellaguarda , Francis A. Farraye , Kelly Y. Chun , Michael Zikry , Monique Bastidas , Ann Firestine , Riley G. Craig , Margie E. Boccieri , Millie D. Long , Michael D. Kappelman
|
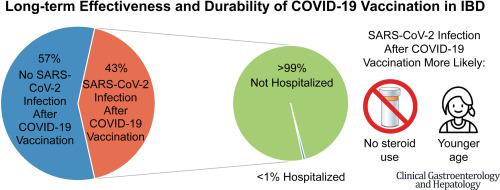
COVID-19 vaccination prevents severe disease in most patients with inflammatory bowel disease (IBD), but immunosuppressive medications can blunt serologic response. We followed adults with IBD for >1 year post–COVID-19 vaccination to describe factors associated with SARS-CoV-2 infection after vaccination, evaluate for a protective SARS-CoV-2 antibody level, characterize SARS-CoV-2 antibody persistence, and identify factors associated with humoral immune response durability. Using a prospective cohort of COVID-19 immunized adults with IBD, we analyzed factors associated with SARS-CoV-2 infection after vaccination. We evaluated for an association between SARS-CoV-2 antibody level 12 weeks postvaccination and subsequent SARS-CoV-2 infection and assessed for a threshold of protection using receiver-operating characteristic curve analysis. We then conducted a separate analysis evaluating factors associated with persistence of SARS-CoV-2 antibodies 52 weeks postimmunization. Almost half (43%) of 1869 participants developed COVID-19 after vaccination, but most infections were mild, and <1% required hospitalization. Older age and corticosteroid use were associated with a decreased risk of SARS-CoV-2 infection postvaccination (50–59 years of age vs 18–29 years of age: adjusted hazard ratio, 0.57; 95% confidence interval, 0.44–0.74; steroid users vs nonusers: adjusted hazard ratio, 0.58; 95% confidence interval, 0.39–0.87). Most (98%) participants had detectable antibody levels at 52 weeks postvaccination. Antibody levels at 12 weeks and number of vaccine doses were positively associated with higher antibody levels at 52 weeks, while anti-tumor necrosis factor α therapy was negatively associated. COVID-19 vaccination generates an effective and durable protective response for the vast majority of adults with IBD, including vulnerable populations such as corticosteroid users and older individuals. Patients with IBD benefit from COVID-19 booster vaccination.
中文翻译:

炎症性肠病患者接种 COVID-19 疫苗的长期有效性和持久性
COVID-19 疫苗接种可以预防大多数炎症性肠病 (IBD) 患者出现严重疾病,但免疫抑制药物可能会减弱血清学反应。我们在接种 COVID-19 疫苗后对患有 IBD 的成年人进行了超过 1 年的跟踪,以描述疫苗接种后与 SARS-CoV-2 感染相关的因素,评估保护性 SARS-CoV-2 抗体水平,表征 SARS-CoV-2 抗体持久性,并确定与体液免疫反应持久性相关的因素。我们利用一组接受过 COVID-19 免疫的 IBD 成人前瞻性队列,分析了疫苗接种后与 SARS-CoV-2 感染相关的因素。我们评估了疫苗接种后 12 周的 SARS-CoV-2 抗体水平与随后的 SARS-CoV-2 感染之间的关联,并使用受试者操作特征曲线分析评估了保护阈值。然后,我们进行了一项单独的分析,评估与免疫后 52 周 SARS-CoV-2 抗体持续存在相关的因素。 1869 名参与者中近一半 (43%) 在接种疫苗后出现了 COVID-19,但大多数感染程度较轻,<1% 需要住院治疗。年龄较大和皮质类固醇的使用与疫苗接种后感染 SARS-CoV-2 的风险降低相关(50-59 岁与 18-29 岁相比:调整后的风险比,0.57;95% 置信区间,0.44-0.74;类固醇用户与非用户:调整后的风险比,0.58;95% 置信区间,0.39–0.87)。大多数 (98%) 参与者在疫苗接种后 52 周时检测到抗体水平。 12周时的抗体水平和疫苗剂量数与52周时较高的抗体水平呈正相关,而抗肿瘤坏死因子α治疗呈负相关。 COVID-19 疫苗接种可为绝大多数 IBD 成年人(包括皮质类固醇使用者和老年人等弱势群体)产生有效且持久的保护反应。 IBD 患者可受益于 COVID-19 加强疫苗接种。
更新日期:2024-02-17
中文翻译:

炎症性肠病患者接种 COVID-19 疫苗的长期有效性和持久性
COVID-19 疫苗接种可以预防大多数炎症性肠病 (IBD) 患者出现严重疾病,但免疫抑制药物可能会减弱血清学反应。我们在接种 COVID-19 疫苗后对患有 IBD 的成年人进行了超过 1 年的跟踪,以描述疫苗接种后与 SARS-CoV-2 感染相关的因素,评估保护性 SARS-CoV-2 抗体水平,表征 SARS-CoV-2 抗体持久性,并确定与体液免疫反应持久性相关的因素。我们利用一组接受过 COVID-19 免疫的 IBD 成人前瞻性队列,分析了疫苗接种后与 SARS-CoV-2 感染相关的因素。我们评估了疫苗接种后 12 周的 SARS-CoV-2 抗体水平与随后的 SARS-CoV-2 感染之间的关联,并使用受试者操作特征曲线分析评估了保护阈值。然后,我们进行了一项单独的分析,评估与免疫后 52 周 SARS-CoV-2 抗体持续存在相关的因素。 1869 名参与者中近一半 (43%) 在接种疫苗后出现了 COVID-19,但大多数感染程度较轻,<1% 需要住院治疗。年龄较大和皮质类固醇的使用与疫苗接种后感染 SARS-CoV-2 的风险降低相关(50-59 岁与 18-29 岁相比:调整后的风险比,0.57;95% 置信区间,0.44-0.74;类固醇用户与非用户:调整后的风险比,0.58;95% 置信区间,0.39–0.87)。大多数 (98%) 参与者在疫苗接种后 52 周时检测到抗体水平。 12周时的抗体水平和疫苗剂量数与52周时较高的抗体水平呈正相关,而抗肿瘤坏死因子α治疗呈负相关。 COVID-19 疫苗接种可为绝大多数 IBD 成年人(包括皮质类固醇使用者和老年人等弱势群体)产生有效且持久的保护反应。 IBD 患者可受益于 COVID-19 加强疫苗接种。



























 京公网安备 11010802027423号
京公网安备 11010802027423号