当前位置:
X-MOL 学术
›
Biophys. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coupling of zinc and GTP binding drives G-domain folding in Acinetobacter baumannii ZigA
Biophysical Journal ( IF 3.4 ) Pub Date : 2024-03-08 , DOI: 10.1016/j.bpj.2024.03.010 Maximillian K. Osterberg , Ally Smith , Courtney Campbell , Daniel Deredge , Timothy L. Stemmler , David P. Giedroc
Biophysical Journal ( IF 3.4 ) Pub Date : 2024-03-08 , DOI: 10.1016/j.bpj.2024.03.010 Maximillian K. Osterberg , Ally Smith , Courtney Campbell , Daniel Deredge , Timothy L. Stemmler , David P. Giedroc
|
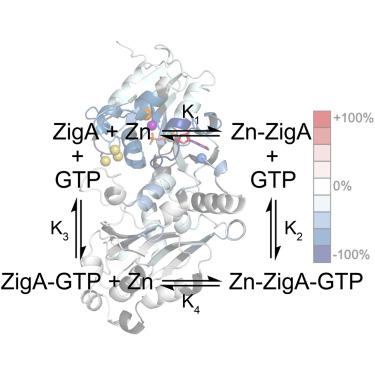
COG0523 proteins, also known as nucleotide-dependent metallochaperones, are a poorly understood class of small P-loop G3E GTPases. Multiple family members play critical roles in bacterial pathogen survival during an infection as part of the adaptive response to host-mediated “nutritional immunity.” Our understanding of the structure, dynamics, and molecular-level function of COG0523 proteins, apart from the eukaryotic homolog, Zng1, remains in its infancy. Here, we use X-ray absorption spectroscopy to establish that () ZigA coordinates Zn using all three cysteines derived from the invariant CXCC motif to form an S(N/O) coordination complex, a feature inconsistent with the Zn-bound crystal structure of a distantly related COG0523 protein of unknown function from , YjiA. The binding of Zn and guanine nucleotides is thermodynamically linked in ZigA, and this linkage is more favorable for the substrate GTP relative to the product GDP. Part of this coupling originates with nucleotide-induced stabilization of the G-domain tertiary structure as revealed by global thermodynamics measurements and hydrogen-deuterium exchange mass spectrometry (HDX-MS). HDX-MS also reveals that the HDX behavior of the G2 (switch 1) loop is highly sensitive to nucleotide status and becomes more exchange labile in the GDP (product)-bound state. Significant long-range perturbation of local stability in both the G-domain and the C-terminal domain define a candidate binding pocket for a client protein that appears sensitive to nucleotide status (GDP versus GTP). We place these new insights into the structure, dynamics, and energetics of intermolecular metal transfer into the context of a model for ZigA metallochaperone function.
中文翻译:

锌和 GTP 结合的偶联驱动鲍曼不动杆菌 ZigA 中的 G 结构域折叠
COG0523 蛋白,也称为核苷酸依赖性金属伴侣,是一类人们知之甚少的小 P 环 G3E GTP 酶。作为对宿主介导的“营养免疫”的适应性反应的一部分,多个家庭成员在感染期间细菌病原体的存活中发挥着关键作用。除了真核同源物 Zng1 之外,我们对 COG0523 蛋白的结构、动力学和分子水平功能的了解仍处于起步阶段。在这里,我们使用 X 射线吸收光谱来确定 () ZigA 使用源自不变 CXCC 基序的所有三个半胱氨酸来协调 Zn,形成 S(N/O) 配位复合物,这一特征与 Zn 结合的晶体结构不一致来自 YjiA 的功能未知的远缘 COG0523 蛋白。 ZigA 中 Zn 和鸟嘌呤核苷酸的结合是热力学连接的,相对于产物 GDP,这种连接更有利于底物 GTP。这种耦合的部分原因在于核苷酸诱导的 G 结构域三级结构的稳定,正如整体热力学测量和氢-氘交换质谱 (HDX-MS) 所揭示的那样。 HDX-MS 还表明,G2(开关 1)环的 HDX 行为对核苷酸状态高度敏感,并且在 GDP(产物)结合状态下变得更加不稳定。 G 结构域和 C 末端结构域局部稳定性的显着远程扰动定义了对核苷酸状态(GDP 与 GTP)敏感的客户蛋白的候选结合袋。我们将这些对分子间金属转移的结构、动力学和能量学的新见解放入 ZigA 金属伴侣功能模型的背景中。
更新日期:2024-03-08
中文翻译:

锌和 GTP 结合的偶联驱动鲍曼不动杆菌 ZigA 中的 G 结构域折叠
COG0523 蛋白,也称为核苷酸依赖性金属伴侣,是一类人们知之甚少的小 P 环 G3E GTP 酶。作为对宿主介导的“营养免疫”的适应性反应的一部分,多个家庭成员在感染期间细菌病原体的存活中发挥着关键作用。除了真核同源物 Zng1 之外,我们对 COG0523 蛋白的结构、动力学和分子水平功能的了解仍处于起步阶段。在这里,我们使用 X 射线吸收光谱来确定 () ZigA 使用源自不变 CXCC 基序的所有三个半胱氨酸来协调 Zn,形成 S(N/O) 配位复合物,这一特征与 Zn 结合的晶体结构不一致来自 YjiA 的功能未知的远缘 COG0523 蛋白。 ZigA 中 Zn 和鸟嘌呤核苷酸的结合是热力学连接的,相对于产物 GDP,这种连接更有利于底物 GTP。这种耦合的部分原因在于核苷酸诱导的 G 结构域三级结构的稳定,正如整体热力学测量和氢-氘交换质谱 (HDX-MS) 所揭示的那样。 HDX-MS 还表明,G2(开关 1)环的 HDX 行为对核苷酸状态高度敏感,并且在 GDP(产物)结合状态下变得更加不稳定。 G 结构域和 C 末端结构域局部稳定性的显着远程扰动定义了对核苷酸状态(GDP 与 GTP)敏感的客户蛋白的候选结合袋。我们将这些对分子间金属转移的结构、动力学和能量学的新见解放入 ZigA 金属伴侣功能模型的背景中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号