The Journal of Prevention of Alzheimer's Disease ( IF 6.4 ) Pub Date : 2024-03-19 , DOI: 10.14283/jpad.2024.60 F.G. Boess , M.A. Scelsi , T. Grimmer , R.J. Perry , M. Tonietto , G. Klein , C. Hofmann , M. Salami , J. Wojtowicz , C.J. Lansdall , C. Lane , G.A. Kerchner , J. Smith , R.S. Doody
|
Background
Monoclonal antibodies that target amyloid-beta and remove amyloid plaques can slow cognitive and functional decline in early Alzheimer’s disease. Gantenerumab is a subcutaneously administered fully-human anti-amyloid-beta monoclonal antibody with highest affinity for aggregated amyloid-beta. Since the phase 3 GRADUATE trials did not meet the primary endpoint (change from baseline to Week 116 in Clinical Dementia Rating scale–Sum of Boxes), development of gantenerumab in sporadic Alzheimer’s disease was stopped and all ongoing trials were terminated early due to sponsor decision. Subcutaneous administration at the clinic or at home by care partner would be an important option for other therapies in this class in order to increase flexibility and reduce overall burden. The insights obtained from the experience with gantenerumab home administration by care partner in the phase 2 GRADUATION trial will serve to guide the ongoing efforts with other anti-amyloid-beta antibodies.
Objectives
To evaluate the pharmacodynamic effects on brain amyloid load of once weekly subcutaneous administration of gantenerumab and the safety and feasibility of home administration by care partners.
Design
Phase 2, open-label, single arm study.
Setting
Multicenter trial conducted in 33 sites in 8 countries from November 2020 to March 2023.
Participants
Participants aged 50 to 90 with early symptomatic Alzheimer’s disease (mild cognitive impairment/ mild dementia due to Alzheimer’s disease), and evidence of amyloid positron emission tomography positivity.
Intervention
Participants could receive up to 255 mg gantenerumab once-weekly, administered subcutaneously at site or at home by healthcare professionals or non-healthcare-professional care partners.
Measurements
The primary endpoint was the change from baseline to Week 52 and to Week 104 in brain amyloid load as measured by PET centiloid levels. The secondary endpoints were responses to the home administration questionnaire, plasma concentrations and safety.
Results
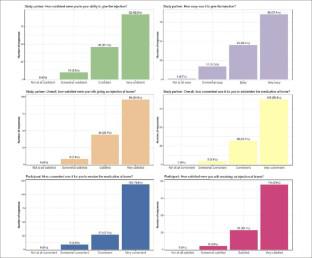
The overall number of participants enrolled was 192, with a mean (standard deviation) amyloid PET load at baseline of 101.80 (29.80) centiloids. At the time of early study termination by sponsor, 149 participants had valid Week 52 amyloid PET data (primary endpoint), and 12 participants had an early termination PET within the pre-defined time range of Week 104. The mean change in amyloid PET from baseline to Week 52 and Week 104 was −26.19 centiloids (range: −75.6–15.8; n=149) and −35.48 centiloids (range: −63.2–−7.0; n=12), respectively. Responses to the home administration questionnaire at Week 52 (n=148) indicated that the majority of care partners (88–97%) considered administration of study drug at home easy (30.4%) or very easy (57.4%), and convenient (25.7%) or very convenient (70.9%). Care partners felt confident (31.1%) or very confident (62.2%) and satisfied (29.7%) or very satisfied (64.9%) with giving the injection at home. Responses by care partners at Week 36 (n=72), Week 76 (n=126) and Week 104 (n=29) and participant (patient) assessment of convenience and satisfaction at these time points were similar. There were no new safety findings associated with gantenerumab administered subcutaneously once weekly at 255 mg or safety issues associated with at-home injections by non-healthcare professional care partners.
Conclusions
Once-weekly subcutaneous home administration of the anti-amyloid-beta antibody gantenerumab by non-healthcare-professional care partners to participants with early Alzheimer’s disease was feasible, safe, well tolerated, and considered as a convenient option by both the care partners and participants with Alzheimer’s disease. Although gantenerumab’s development has been stopped due to lack of efficacy, this approach has the potential to reduce the frequency of hospital/outpatient clinic visits required for treatment with other anti-amyloid-β antibodies and can increase flexibility of drug administration for people living with Alzheimer’s disease and their families.
中文翻译:

护理伙伴对早期阿尔茨海默病患者在家给予 Gantenerumab:可行性、安全性和药效影响
背景
靶向β-淀粉样蛋白并去除淀粉样蛋白斑块的单克隆抗体可以减缓早期阿尔茨海默病的认知和功能下降。Gantenerumab 是一种皮下注射的全人源抗淀粉样蛋白-β 单克隆抗体,对聚集的淀粉样蛋白-β 具有最高的亲和力。由于 3 期 GRADUATE 试验未达到主要终点(临床痴呆评定量表从基线更改为第 116 周——框总和),因此停止了 gantenerumab 治疗散发性阿尔茨海默病的开发,并且由于申办者的决定,所有正在进行的试验都提前终止。在诊所或由护理伙伴在家进行皮下注射将是此类其他疗法的重要选择,以提高灵活性并减轻总体负担。在 2 期毕业试验中,护理伙伴从 gantenerumab 家庭给药经验中获得的见解将有助于指导其他抗淀粉样蛋白 β 抗体的持续努力。
目标
评估每周一次皮下注射 gantenerumab 对脑淀粉样蛋白负荷的药效学影响,以及护理伙伴在家给药的安全性和可行性。
设计
第二阶段,开放标签,单臂研究。
环境
2020年11月至2023年3月在8个国家的33个地点进行多中心试验。
参加者
年龄在 50 岁至 90 岁之间、患有早期症状性阿尔茨海默病(阿尔茨海默病导致的轻度认知障碍/轻度痴呆)的参与者,以及淀粉样蛋白正电子发射断层扫描呈阳性的证据。
干涉
参与者每周可以接受一次最多 255 毫克的gantenerumab,由医疗保健专业人员或非医疗保健专业护理合作伙伴在现场或家中皮下注射。
测量
主要终点是通过 PET Centiloid 水平测量的脑淀粉样蛋白负荷从基线到第 52 周和第 104 周的变化。次要终点是对家庭给药问卷、血浆浓度和安全性的回答。
结果
入组的参与者总数为 192 人,基线时淀粉样蛋白 PET 负荷的平均(标准差)为 101.80 (29.80) 个 centiloids。在申办者提前终止研究时,149 名参与者拥有有效的第 52 周淀粉样蛋白 PET 数据(主要终点),12 名参与者在第 104 周的预定时间范围内进行了提前终止 PET。淀粉样蛋白 PET 的平均变化为第52周和第104周的基线分别为-26.19厘粒数(范围:-75.6-15.8;n=149)和-35.48厘粒数(范围:-63.2--7.0;n=12)。第 52 周时对家庭给药问卷的答复 (n=148) 表明,大多数护理伙伴 (88-97%) 认为在家给药研究药物很容易 (30.4%) 或非常容易 (57.4%),并且方便 ( 25.7%)或非常方便(70.9%)。护理伙伴对在家注射感到有信心(31.1%)或非常有信心(62.2%)以及满意(29.7%)或非常满意(64.9%)。护理合作伙伴在第 36 周 (n=72)、第 76 周 (n=126) 和第 104 周 (n=29) 的反应以及参与者(患者)在这些时间点对便利性和满意度的评估相似。没有与每周一次皮下注射 255 毫克甘特鲁单抗相关的新安全性发现,也没有与非医疗保健专业护理合作伙伴在家注射相关的安全性问题。
结论
非医疗保健专业护理合作伙伴向患有早期阿尔茨海默氏病的参与者每周一次皮下注射抗淀粉样蛋白 β 抗体 gantenerumab 是可行的、安全的、耐受性良好,并且被护理合作伙伴和参与者认为是一种方便的选择患有阿尔茨海默病。尽管 gantenerumab 的开发因缺乏疗效而停止,但这种方法有可能减少其他抗淀粉样蛋白 β 抗体治疗所需的医院/门诊就诊频率,并可以提高阿尔茨海默病患者用药的灵活性疾病和他们的家人。



























 京公网安备 11010802027423号
京公网安备 11010802027423号