Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-15 , DOI: 10.3762/bjoc.20.52 Ran Zou , Xin Li , Xiaochen Chen , Yue-Wei Guo , Baofu Xu
Abstract
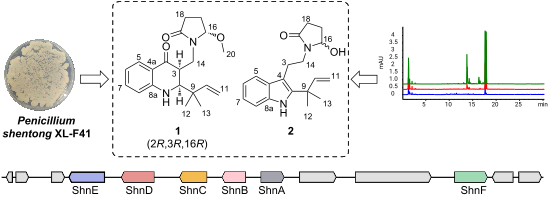
Penicillium strains are renowned for producing diverse secondary metabolites with unique structures and promising bioactivities. Our chemical investigations, accompanied by fermentation media optimization, of a newly isolated fungus, Penicillium shentong XL-F41, led to the isolation of twelve compounds. Among these are two novel indole terpene alkaloids, shentonins A and B (1 and 2), and a new fatty acid 3. Shentonin A (1) is distinguished by an unusual methyl modification at the oxygen atom of the typical succinimide ring, a feature not seen in the structurally similar brocaeloid D. Additionally, shentonin A (1) exhibits a cis relationship between H-3 and H-4, as opposed to the trans configuration in brocaeloid D, suggesting a divergent enzymatic ring-expansion process in their respective fungi. Both shentonins A (1) and B (2) also feature a reduction of a carbonyl to a hydroxy group within the succinimide ring. All isolated compounds were subjected to antimicrobial evaluations, and compound 12 was found to have moderate inhibitory activity against Candia albicans. Moreover, genome sequencing of Penicillium shentong XL-F41 uncovered abundant silent biosynthetic gene clusters, indicating the need for future efforts to activate these clusters and unlock the full chemical potential of the fungus.
Beilstein J. Org. Chem. 2024, 20, 597–606. doi:10.3762/bjoc.20.52
中文翻译:

神通青霉XL-F41的化学和生物合成潜力
摘要
青霉菌菌株以产生具有独特结构和有前景的生物活性的多种次生代谢物而闻名。我们对新分离的真菌Penicillium Shentong XL-F41 进行化学研究,并结合发酵培养基优化,分离出 12 种化合物。其中包括两种新型吲哚萜生物碱,神通宁 A 和 B(1和2),以及一种新脂肪酸3。Shentonin A ( 1 ) 的特点是典型琥珀酰亚胺环的氧原子上有一个不寻常的甲基修饰,这一特征在结构相似的 brocaeloid D 中未见。此外, Shentonin A ( 1 ) 在 H-3 和 H 之间表现出顺式关系-4,与 brocaeloid D 中的反式构型相反,表明它们各自的真菌中存在不同的酶促扩环过程。申通宁 A ( 1 ) 和 B ( 2 ) 还具有将琥珀酰亚胺环内的羰基还原为羟基的特征。所有分离的化合物均经过抗菌评估,发现化合物12对白色念珠菌具有中等抑制活性。此外,Penicillium Shentong XL-F41 的基因组测序发现了丰富的沉默生物合成基因簇,表明未来需要努力激活这些簇并释放真菌的全部化学潜力。

贝尔斯坦 J. 组织。化学。 2024, 20, 597–606。doi:10.3762/bjoc.20.52



























 京公网安备 11010802027423号
京公网安备 11010802027423号