当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ion Spectroscopy Reveals Structural Difference for Proteins Microhydrated by Retention and Condensation of Water
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.jpca.4c00263 Andrei Zviagin 1 , Oleg V. Boyarkin 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.jpca.4c00263 Andrei Zviagin 1 , Oleg V. Boyarkin 1
Affiliation
|
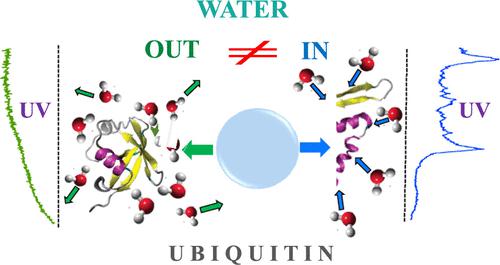
Protein ubiquitin in its +7 charge state microhydrated by 5 and 10 water molecules has been interrogated in the gas phase by cold ion UV/IR spectroscopy. The complexes were formed either by condensing water onto the unfolded bare proteins in a temperature-controlled ion trap or by incomplete dehydration of the folded proteins. In the case of cryogenic condensation, the UV spectra of the complexes exhibit a resolved vibrational structure, which looks similar to the spectrum of bare unfolded ubiquitin. The spectra become, however, broad-band with no structure when complexes of the same size are produced by incomplete dehydration under soft conditions of electrospray ionization. We attribute this spectroscopic dissimilarity to the structural difference of the protein: condensing a few water molecules cannot refold the gas-phase structure of the bare ubiquitin, while the retained water preserves its solution-like folded motif through evaporative cooling. This assessment is firmly confirmed by IR spectroscopy, which reveals the presence of free NH and carboxylic OH stretching vibrations only in the complexes with condensed water.
中文翻译:

离子光谱揭示了通过水的保留和冷凝而微水化的蛋白质的结构差异
由 5 和 10 个水分子微水合的 +7 电荷状态的蛋白质泛素已在气相中通过冷离子 UV/IR 光谱进行了研究。这些复合物是通过在温控离子阱中将水冷凝到未折叠的裸蛋白上或通过折叠蛋白的不完全脱水而形成的。在低温冷凝的情况下,复合物的紫外光谱表现出解析的振动结构,看起来类似于裸露的未折叠泛素的光谱。然而,当在电喷雾电离的软条件下不完全脱水产生相同尺寸的配合物时,光谱变得没有结构的宽带。我们将这种光谱差异归因于蛋白质的结构差异:冷凝一些水分子无法重新折叠裸泛素的气相结构,而保留的水通过蒸发冷却保留其溶液状折叠基序。这一评估得到了红外光谱的证实,红外光谱揭示了仅在与缩合水的复合物中存在游离NH和羧基OH伸缩振动。
更新日期:2024-03-15
中文翻译:

离子光谱揭示了通过水的保留和冷凝而微水化的蛋白质的结构差异
由 5 和 10 个水分子微水合的 +7 电荷状态的蛋白质泛素已在气相中通过冷离子 UV/IR 光谱进行了研究。这些复合物是通过在温控离子阱中将水冷凝到未折叠的裸蛋白上或通过折叠蛋白的不完全脱水而形成的。在低温冷凝的情况下,复合物的紫外光谱表现出解析的振动结构,看起来类似于裸露的未折叠泛素的光谱。然而,当在电喷雾电离的软条件下不完全脱水产生相同尺寸的配合物时,光谱变得没有结构的宽带。我们将这种光谱差异归因于蛋白质的结构差异:冷凝一些水分子无法重新折叠裸泛素的气相结构,而保留的水通过蒸发冷却保留其溶液状折叠基序。这一评估得到了红外光谱的证实,红外光谱揭示了仅在与缩合水的复合物中存在游离NH和羧基OH伸缩振动。



























 京公网安备 11010802027423号
京公网安备 11010802027423号