Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In vitro detection of circulating tumor cells using the nicking endonuclease-assisted lanthanide metal luminescence amplification strategy
Talanta ( IF 6.1 ) Pub Date : 2024-03-14 , DOI: 10.1016/j.talanta.2024.125909 Xuekang Wang , Yating Zeng , Nanhang Zhu , Yue Yu , Qiangying Yi , Yao Wu
Talanta ( IF 6.1 ) Pub Date : 2024-03-14 , DOI: 10.1016/j.talanta.2024.125909 Xuekang Wang , Yating Zeng , Nanhang Zhu , Yue Yu , Qiangying Yi , Yao Wu
|
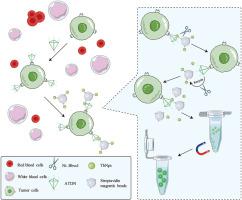
The in vitro detection of circulating tumor cells (CTCs) has been proven as a vital method for early diagnosis and evaluation of cancer metastasis, since the existence and number fluctuation of CTCs have shown close correlation with clinical outcomes. However, it remains difficult and technically challenging to realize accurate CTCs detection, due to the rarity of CTCs in the blood samples with complex components. Herein, we reported a CTCs in vitro detection strategy, utilizing a loop amplification strategy based on DNA tetrahedron and nicking endonuclease reaction, as well as the anti-background interference based on lanthanide metal luminescence strategy. In this work, a detection system (ATDN-MLLPs) composed of an aptamer-functionalized tetrahedral DNA nanostructure (ATDN) and magnetic lanthanide luminescent particles (MLLPs) was developed. ATDN targeted the tumor cells via aptamer-antigen recognition and extended three hybridizable target DNA segments from the apex of a DNA tetrahedron to pair with probe DNA on MLLPs. Then, the nicking endonuclease (Nt.BbvCI) recognized the formed double-strand DNA and nicked the probe DNA to release the target DNA for recycling, and the released TbNps served as a high signal-to-noise ratio fluorescence signal source for CTCs detection. With a detection limit of 5 cells/mL, CTCs were selectively screened throughout a linear response range of low orders of magnitude. In addition, the ATDN-MLLPs system was attempted to detect possible existence of CTCs in biological samples in vitro.
中文翻译:

使用切口核酸内切酶辅助的镧系金属发光扩增策略体外检测循环肿瘤细胞
循环肿瘤细胞(CTC)的体外检测已被证明是早期诊断和评估癌症转移的重要方法,因为CTC的存在和数量波动与临床结果密切相关。然而,由于成分复杂的血液样本中CTC的稀有性,实现准确的CTC检测仍然存在困难和技术挑战。在此,我们报道了一种CTC体外检测策略,利用基于DNA四面体和切口核酸内切酶反应的环扩增策略,以及基于镧系金属发光策略的抗背景干扰策略。在这项工作中,开发了一种由适配体功能化四面体DNA纳米结构(ATDN)和磁性镧系元素发光颗粒(MLLP)组成的检测系统(ATDN-MLLP)。 ATDN 通过适配体抗原识别来靶向肿瘤细胞,并从 DNA 四面体的顶点延伸三个可杂交的目标 DNA 片段,以与 MLLP 上的探针 DNA 配对。然后,切口核酸内切酶(Nt.BbvCI)识别形成的双链DNA,并对探针DNA进行切口,释放出目标DNA进行回收,释放的TbNps作为CTC检测的高信噪比荧光信号源。检测限为 5 个细胞/mL,在低数量级的线性响应范围内选择性筛选 CTC。此外,ATDN-MLLPs系统还尝试在体外检测生物样品中可能存在的CTC。
更新日期:2024-03-14
中文翻译:

使用切口核酸内切酶辅助的镧系金属发光扩增策略体外检测循环肿瘤细胞
循环肿瘤细胞(CTC)的体外检测已被证明是早期诊断和评估癌症转移的重要方法,因为CTC的存在和数量波动与临床结果密切相关。然而,由于成分复杂的血液样本中CTC的稀有性,实现准确的CTC检测仍然存在困难和技术挑战。在此,我们报道了一种CTC体外检测策略,利用基于DNA四面体和切口核酸内切酶反应的环扩增策略,以及基于镧系金属发光策略的抗背景干扰策略。在这项工作中,开发了一种由适配体功能化四面体DNA纳米结构(ATDN)和磁性镧系元素发光颗粒(MLLP)组成的检测系统(ATDN-MLLP)。 ATDN 通过适配体抗原识别来靶向肿瘤细胞,并从 DNA 四面体的顶点延伸三个可杂交的目标 DNA 片段,以与 MLLP 上的探针 DNA 配对。然后,切口核酸内切酶(Nt.BbvCI)识别形成的双链DNA,并对探针DNA进行切口,释放出目标DNA进行回收,释放的TbNps作为CTC检测的高信噪比荧光信号源。检测限为 5 个细胞/mL,在低数量级的线性响应范围内选择性筛选 CTC。此外,ATDN-MLLPs系统还尝试在体外检测生物样品中可能存在的CTC。



























 京公网安备 11010802027423号
京公网安备 11010802027423号