当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of spiro[piperidine-4,3′-pyrrolo[3,4-c]quinolines via 1,3-dipolar cycloaddition of azomethine ylides and 3-Nitro-2(1H)-quinolones
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2024-03-13 , DOI: 10.1016/j.tetlet.2024.155001 Márk Molnár , Tamás Gáti , Attila Bényei , Miklós Nyerges
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2024-03-13 , DOI: 10.1016/j.tetlet.2024.155001 Márk Molnár , Tamás Gáti , Attila Bényei , Miklós Nyerges
|
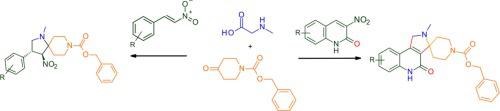
The 1,3-dipolar cycloaddition of 3-nitro-2(1)-quinolones and β-nitro-styrenes with azomethine ylides derived from sarcosine and benzyl 4-oxopiperidine-1-carboxylate have been investigated. The structure of the formed cycloadducts were studied in detail by X-ray diffraction and NMR spectroscopy methods. This process is a solid and versatile strategy for rapidly assembling quinoline fused pyrrolines scaffold with a spiropiperidine part containing orthogonal diversification points for further substitutions.
中文翻译:

通过偶氮甲碱叶立德和 3-硝基-2(1H)-喹诺酮类化合物的 1,3-偶极环加成反应合成螺[哌啶-4,3'-吡咯并[3,4-c]喹啉
研究了 3-硝基-2(1)-喹诺酮类和 β-硝基-苯乙烯与衍生自肌氨酸和 4-氧代哌啶-1-甲酸苄酯的甲亚胺叶立德的 1,3-偶极环加成反应。通过X射线衍射和核磁共振波谱方法详细研究了所形成的环加合物的结构。该过程是一种可靠且通用的策略,用于快速组装喹啉融合吡咯啉支架与含有正交多样化点以进行进一步取代的螺哌啶部分。
更新日期:2024-03-13
中文翻译:

通过偶氮甲碱叶立德和 3-硝基-2(1H)-喹诺酮类化合物的 1,3-偶极环加成反应合成螺[哌啶-4,3'-吡咯并[3,4-c]喹啉
研究了 3-硝基-2(1)-喹诺酮类和 β-硝基-苯乙烯与衍生自肌氨酸和 4-氧代哌啶-1-甲酸苄酯的甲亚胺叶立德的 1,3-偶极环加成反应。通过X射线衍射和核磁共振波谱方法详细研究了所形成的环加合物的结构。该过程是一种可靠且通用的策略,用于快速组装喹啉融合吡咯啉支架与含有正交多样化点以进行进一步取代的螺哌啶部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号