当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Determination and Thermodynamic Model Analysis of Esculetin in Different Solvents from 273.15 to 318.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.jced.3c00771 Cheng Yang 1 , Haijun Yan 2 , Qianhe Huang 3 , Wenge Yang 1 , Yonghong Hu 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-15 , DOI: 10.1021/acs.jced.3c00771 Cheng Yang 1 , Haijun Yan 2 , Qianhe Huang 3 , Wenge Yang 1 , Yonghong Hu 3
Affiliation
|
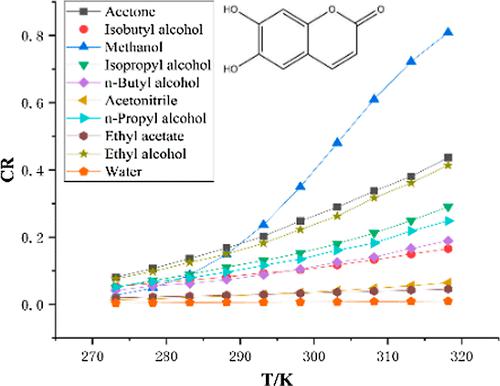
Esculetin is derived from the leaves of lemons of Rutaceae and the bark of bittersweet ash, belladonna, mandala, and Rehmannia plants. In this research, the solubility of esculetin in ten solvents such as ethyl acetate, isobutyl alcohol, water, methanol, acetonitrile, n-propyl alcohol, isopropyl alcohol, n-butanol, ethanol, and acetone was determined by utilizing static equilibrium-high performance liquid chromatography (HPLC) under standard atmospheric pressure conditions and temperatures in the range of 273.15 to 318.15 K. Among the above ten pure solvents, water displayed the least solubility, while methanol exhibited the highest solubility. At the same temperature (T = 273.15 ∼ 318.15 K), the solubility of esculetin increased with the increase of the molar ratio of soluble solvents. Similarly, when the temperature increases (T = 273.15 ∼ 318.15 K), the solubility of the three binary solvents increases under the condition of a constant molar ratio. The above results suggest that the main factor affecting the solubility of esculetin in the solvent may be the polarization/bipolarity of the solvent. The experimental data was fitted by five thermodynamic models (the Buchowski–Ksiazaczak λh model, the Modified Apelblat model, the Jouyban–Acree model, the SUN model, and the CNIBS/R-K model), and the relative average deviation and root-mean-square deviation of the data were calculated, which proved that the correlation of the solubility data and the five models was relatively good. XRD and DSC were used to detect the crystal form and stability of esculetin during the experiment.
中文翻译:

七叶亭在273.15~318.15 K不同溶剂中的溶解度测定及热力学模型分析
七叶亭提取自芸香科柠檬叶和苦乐参半的白蜡树、颠茄、曼陀罗和地黄植物的树皮。本研究采用静态平衡高效法测定了七叶亭在乙酸乙酯、异丁醇、水、甲醇、乙腈、正丙醇、异丙醇、正丁醇、乙醇、丙酮等10种溶剂中的溶解度。在标准大气压条件和温度273.15至318.15 K范围内进行液相色谱分析。在上述10种纯溶剂中,水的溶解度最小,而甲醇的溶解度最高。相同温度(T =273.15∼318.15 K)下,七叶亭的溶解度随着可溶溶剂摩尔比的增加而增加。同样,当温度升高(T = 273.15 ∼ 318.15 K)时,在摩尔比恒定的情况下,三种二元溶剂的溶解度增加。上述结果表明,影响七叶亭在溶剂中溶解度的主要因素可能是溶剂的极化/双极性。实验数据通过五个热力学模型(Buchowski-Ksiazaczak λ h模型、Modified Apelblat 模型、Jouyban-Acree 模型、SUN 模型和 CNIBS/RK 模型)进行拟合,并得到相对平均偏差和均方根计算数据的平方偏差,证明溶解度数据与五个模型的相关性较好。实验过程中采用XRD和DSC对七叶亭的晶型及稳定性进行了检测。
更新日期:2024-03-15
中文翻译:

七叶亭在273.15~318.15 K不同溶剂中的溶解度测定及热力学模型分析
七叶亭提取自芸香科柠檬叶和苦乐参半的白蜡树、颠茄、曼陀罗和地黄植物的树皮。本研究采用静态平衡高效法测定了七叶亭在乙酸乙酯、异丁醇、水、甲醇、乙腈、正丙醇、异丙醇、正丁醇、乙醇、丙酮等10种溶剂中的溶解度。在标准大气压条件和温度273.15至318.15 K范围内进行液相色谱分析。在上述10种纯溶剂中,水的溶解度最小,而甲醇的溶解度最高。相同温度(T =273.15∼318.15 K)下,七叶亭的溶解度随着可溶溶剂摩尔比的增加而增加。同样,当温度升高(T = 273.15 ∼ 318.15 K)时,在摩尔比恒定的情况下,三种二元溶剂的溶解度增加。上述结果表明,影响七叶亭在溶剂中溶解度的主要因素可能是溶剂的极化/双极性。实验数据通过五个热力学模型(Buchowski-Ksiazaczak λ h模型、Modified Apelblat 模型、Jouyban-Acree 模型、SUN 模型和 CNIBS/RK 模型)进行拟合,并得到相对平均偏差和均方根计算数据的平方偏差,证明溶解度数据与五个模型的相关性较好。实验过程中采用XRD和DSC对七叶亭的晶型及稳定性进行了检测。



























 京公网安备 11010802027423号
京公网安备 11010802027423号