当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A laterally-fused N-heterocyclic carbene framework from polysubstituted aminoimidazo[5,1-b]oxazol-6-ium salts
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-18 , DOI: 10.3762/bjoc.20.54 Andrew D Gillie , Matthew G Wakeling , Bethan L Greene , Louise Male , Paul W Davies
中文翻译:

来自多取代氨基咪唑并[5,1-b]恶唑-6-鎓盐的横向稠合N-杂环卡宾骨架
更新日期:2024-03-18
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2024-03-18 , DOI: 10.3762/bjoc.20.54 Andrew D Gillie , Matthew G Wakeling , Bethan L Greene , Louise Male , Paul W Davies
Abstract
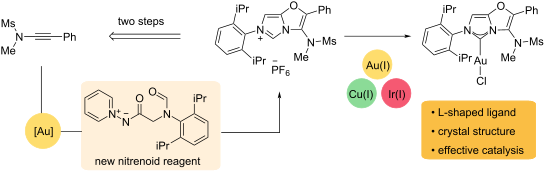
A polysubstituted 3-aminoimidazo[5,1-b]oxazol-6-ium framework has been accessed from a new nitrenoid reagent by a two-step ynamide annulation and imidazolium ring-formation sequence. Metalation with Au(I), Cu(I) and Ir(I) at the C2 position provides an L-shaped NHC ligand scaffold that has been validated in gold-catalysed alkyne hydration and arylative cyclisation reactions.
Beilstein J. Org. Chem. 2024, 20, 621–627. doi:10.3762/bjoc.20.54
中文翻译:

来自多取代氨基咪唑并[5,1-b]恶唑-6-鎓盐的横向稠合N-杂环卡宾骨架
摘要
通过两步炔酰胺成环和咪唑鎓环形成序列,从新型氮烯类试剂中获得了多取代的3-氨基咪唑并[5,1- b ]恶唑-6-鎓骨架。C2 位上的 Au(I)、Cu(I) 和 Ir(I) 金属化提供了 L 形 NHC 配体支架,该支架已在金催化的炔水合和芳基化环化反应中得到验证。

贝尔斯坦 J. 组织。化学。 2024, 20, 621–627。doi:10.3762/bjoc.20.54



























 京公网安备 11010802027423号
京公网安备 11010802027423号