当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
AQP4-DARPin1: A Chimeric Antigen Based on Scaffold Protein DARPin for Efficient Detection of AQP4-IgG in NMOSD
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.biochem.3c00688 Xiaofei Wang 1 , Shubei Ma 2 , Ying Bai 3 , Xinyang Wu 1 , Fangling Ji 1 , Lingyun Jia 1
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.biochem.3c00688 Xiaofei Wang 1 , Shubei Ma 2 , Ying Bai 3 , Xinyang Wu 1 , Fangling Ji 1 , Lingyun Jia 1
Affiliation
|
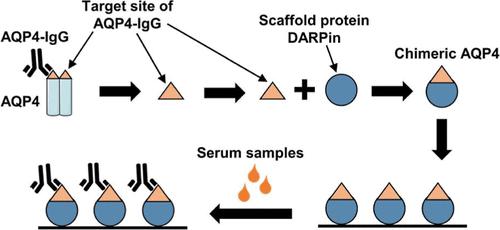
AQP4-IgG is an autoantibody associated with neuromyelitis optica spectroscopic disorder (NMOSD), a central nervous system inflammatory disease that requires early diagnosis and treatment. We designed two fusion proteins, AQP4-DARPin1 and AQP4-DARPin2, comprising the complete antigenic epitopes of aquaporin-4 (AQP4) and the constant region of the scaffold protein DARPin. These fusion proteins were expressed and purified from Escherichia coli and coated on microplates to develop an efficient method for detecting AQP4-IgG. Molecular dynamics simulation revealed that the fusion of AQP4 extracellular epitopes with DARPin did not alter the main structure of DARPin. The purified AQP4-DARPins bound recombinant antibody rAb-53 (AQP4-IgG) with affinities of 135 and 285 nM, respectively. Enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation demonstrated that AQP4-DARPin1 specifically recognized AQP4-IgG in the NMOSD patient serum. AQP4-DARPin1 as a coated antigen showed higher ELISA signal and end point dilution ratio than full-length AQP4. Our AQP4-DARPin1-coated AQP4-IgG ELISA had 100% specificity and 90% sensitivity. These results indicate that AQP4-DARPin1, compared to existing detection strategies that use full-length or extracellular loop peptides of AQP4, provides a new and more effective approach to the ELISA detection of NMOSD.
中文翻译:

AQP4-DARPin1:基于支架蛋白 DARPin 的嵌合抗原,可有效检测 NMOSD 中的 AQP4-IgG
AQP4-IgG 是一种与视神经脊髓炎光谱障碍 (NMOSD) 相关的自身抗体,这是一种需要早期诊断和治疗的中枢神经系统炎症性疾病。我们设计了两种融合蛋白 AQP4-DARPin1 和 AQP4-DARPin2,包含水通道蛋白 4 (AQP4) 的完整抗原表位和支架蛋白 DARPin 的恒定区。这些融合蛋白从大肠杆菌中表达和纯化,并包被在微孔板上,以开发检测 AQP4-IgG 的有效方法。分子动力学模拟表明,AQP4胞外表位与DARPin的融合并没有改变DARPin的主要结构。纯化的 AQP4-DARPins 结合重组抗体 rAb-53 (AQP4-IgG),亲和力分别为 135 和 285 nM。酶联免疫吸附测定 (ELISA) 和免疫沉淀表明 AQP4-DARPin1 特异性识别 NMOSD 患者血清中的 AQP4-IgG。 AQP4-DARPin1 作为包被抗原,显示出比全长 AQP4 更高的 ELISA 信号和终点稀释比。我们的 AQP4-DARPin1 包被的 AQP4-IgG ELISA 具有 100% 的特异性和 90% 的敏感性。这些结果表明,与现有使用AQP4全长或胞外环肽的检测策略相比,AQP4-DARPin1为NMOSD的ELISA检测提供了一种新的、更有效的方法。
更新日期:2024-03-18
中文翻译:

AQP4-DARPin1:基于支架蛋白 DARPin 的嵌合抗原,可有效检测 NMOSD 中的 AQP4-IgG
AQP4-IgG 是一种与视神经脊髓炎光谱障碍 (NMOSD) 相关的自身抗体,这是一种需要早期诊断和治疗的中枢神经系统炎症性疾病。我们设计了两种融合蛋白 AQP4-DARPin1 和 AQP4-DARPin2,包含水通道蛋白 4 (AQP4) 的完整抗原表位和支架蛋白 DARPin 的恒定区。这些融合蛋白从大肠杆菌中表达和纯化,并包被在微孔板上,以开发检测 AQP4-IgG 的有效方法。分子动力学模拟表明,AQP4胞外表位与DARPin的融合并没有改变DARPin的主要结构。纯化的 AQP4-DARPins 结合重组抗体 rAb-53 (AQP4-IgG),亲和力分别为 135 和 285 nM。酶联免疫吸附测定 (ELISA) 和免疫沉淀表明 AQP4-DARPin1 特异性识别 NMOSD 患者血清中的 AQP4-IgG。 AQP4-DARPin1 作为包被抗原,显示出比全长 AQP4 更高的 ELISA 信号和终点稀释比。我们的 AQP4-DARPin1 包被的 AQP4-IgG ELISA 具有 100% 的特异性和 90% 的敏感性。这些结果表明,与现有使用AQP4全长或胞外环肽的检测策略相比,AQP4-DARPin1为NMOSD的ELISA检测提供了一种新的、更有效的方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号