当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tryptophan-Centric Bioinformatics Identifies New Lasso Peptide Modifications
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.biochem.4c00035 Lonnie A. Harris 1 , Hamada Saad 1 , Kyle E. Shelton 1 , Lingyang Zhu 2 , Xiaorui Guo 1 , Douglas A. Mitchell 1, 3, 4
Biochemistry ( IF 2.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.biochem.4c00035 Lonnie A. Harris 1 , Hamada Saad 1 , Kyle E. Shelton 1 , Lingyang Zhu 2 , Xiaorui Guo 1 , Douglas A. Mitchell 1, 3, 4
Affiliation
|
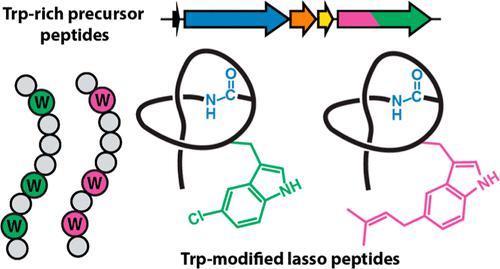
Lasso peptides are a class of ribosomally synthesized and post-translationally modified peptides (RiPPs) defined by a macrolactam linkage between the N-terminus and the side chain of an internal aspartic acid or glutamic acid residue. Instead of adopting a branched-cyclic conformation, lasso peptides are “threaded”, with the C-terminal tail passing through the macrocycle to present a kinetically trapped rotaxane conformation. The availability of enhanced bioinformatics methods has led to a significant increase in the number of secondary modifications found on lasso peptides. To uncover new ancillary modifications in a targeted manner, a bioinformatic strategy was developed to discover lasso peptides with modifications to tryptophan. This effort identified numerous putative lasso peptide biosynthetic gene clusters with core regions of the precursor peptides enriched in tryptophan. Parsing of these tryptophan (Trp)-rich biosynthetic gene clusters uncovered several putative ancillary modifying enzymes, including halogenases and dimethylallyltransferases expected to act upon Trp. Characterization of two gene products yielded a lasso peptide with two 5-Cl-Trp modifications (chlorolassin) and another bearing 5-dimethylallyl-Trp and 2,3-didehydro-Tyr modifications (wygwalassin). Bioinformatic analysis of the requisite halogenase and dimethylallyltransferase revealed numerous other putative Trp-modified lasso peptides that remain uncharacterized. We anticipate that the Trp-centric strategy reported herein may be useful in discovering ancillary modifications for other RiPP classes and, more generally, guide the functional prediction of enzymes that act on specific amino acids.
中文翻译:

以色氨酸为中心的生物信息学鉴定出新的套索肽修饰
Lasso 肽是一类核糖体合成和翻译后修饰的肽 (RiPP),由内部天冬氨酸或谷氨酸残基的 N 末端和侧链之间的大环内酰胺键定义。套索肽没有采用支链环状构象,而是“螺纹化”,C端尾部穿过大环,呈现动力学捕获的轮烷构象。增强生物信息学方法的可用性导致套索肽上发现的二次修饰数量显着增加。为了有针对性地发现新的辅助修饰,开发了一种生物信息学策略来发现色氨酸修饰的套索肽。这项工作鉴定了许多假定的套索肽生物合成基因簇,其中前体肽的核心区域富含色氨酸。对这些富含色氨酸 (Trp) 的生物合成基因簇的解析发现了几种假定的辅助修饰酶,包括预期作用于色氨酸的卤化酶和二甲基烯丙基转移酶。两个基因产物的表征产生了具有两个 5-Cl-Trp 修饰的套索肽(Chlorlassin)和另一个具有 5-二甲基烯丙基-Trp 和 2,3-二脱氢-Tyr 修饰的套索肽(wygwalassin)。对必需的卤化酶和二甲基烯丙基转移酶的生物信息分析揭示了许多其他尚未表征的推定 Trp 修饰的套索肽。我们预计本文报道的以色氨酸为中心的策略可能有助于发现其他 RiPP 类别的辅助修饰,更一般地说,指导作用于特定氨基酸的酶的功能预测。
更新日期:2024-03-18
中文翻译:

以色氨酸为中心的生物信息学鉴定出新的套索肽修饰
Lasso 肽是一类核糖体合成和翻译后修饰的肽 (RiPP),由内部天冬氨酸或谷氨酸残基的 N 末端和侧链之间的大环内酰胺键定义。套索肽没有采用支链环状构象,而是“螺纹化”,C端尾部穿过大环,呈现动力学捕获的轮烷构象。增强生物信息学方法的可用性导致套索肽上发现的二次修饰数量显着增加。为了有针对性地发现新的辅助修饰,开发了一种生物信息学策略来发现色氨酸修饰的套索肽。这项工作鉴定了许多假定的套索肽生物合成基因簇,其中前体肽的核心区域富含色氨酸。对这些富含色氨酸 (Trp) 的生物合成基因簇的解析发现了几种假定的辅助修饰酶,包括预期作用于色氨酸的卤化酶和二甲基烯丙基转移酶。两个基因产物的表征产生了具有两个 5-Cl-Trp 修饰的套索肽(Chlorlassin)和另一个具有 5-二甲基烯丙基-Trp 和 2,3-二脱氢-Tyr 修饰的套索肽(wygwalassin)。对必需的卤化酶和二甲基烯丙基转移酶的生物信息分析揭示了许多其他尚未表征的推定 Trp 修饰的套索肽。我们预计本文报道的以色氨酸为中心的策略可能有助于发现其他 RiPP 类别的辅助修饰,更一般地说,指导作用于特定氨基酸的酶的功能预测。



























 京公网安备 11010802027423号
京公网安备 11010802027423号