当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Non-IRC Mechanism of Bimolecular Reactions with Submerged Barriers: A Case Study of Si+ + H2O Reaction
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.jpca.4c00787 Ruilin Li 1 , Tengyu Gao 1 , Ping Zhang 1 , Anyang Li 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2024-03-18 , DOI: 10.1021/acs.jpca.4c00787 Ruilin Li 1 , Tengyu Gao 1 , Ping Zhang 1 , Anyang Li 1
Affiliation
|
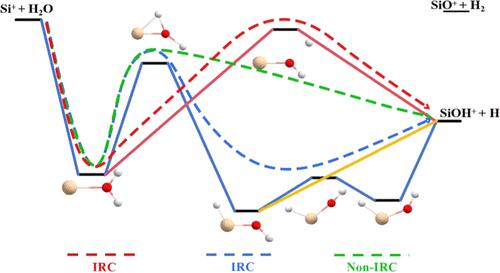
Chemical reactions with submerged barriers may feature interesting dynamic behaviors that are distinct from those with substantial barriers or those entirely dominated by capture. The Si+ + H2O reaction is a prototypical example, involving even two submerged saddle points along the reaction path: one for the direct dissociation of H (H-dissociation SP) and another for H migration from the O-side to the Si-side (H-migration SP). We investigated the intricacies of this process by employing quasi-classical trajectory calculations on an accurate, full-dimensional ab initio potential energy surface. Through careful trajectory analysis, an interesting nonintrinsic reaction coordinate mechanism was found to play an important role in producing SiOH+ and H. This new pathway is featured as that the H atoms do not form HSiOH+ complexes along the minimum-energy path but directly dissociate into the products after passing through the H-migration SP. Furthermore, based on artificially scaled potential energy surfaces (PESs), the impact of barrier height on the reaction is also explored. This work provides new insights into the dynamics of the Si+ + H2O reaction and enriches our understanding of reactions with submerged barriers.
中文翻译:

浸没势垒双分子反应的非 IRC 机理:以 Si+ + H2O 反应为例
水下屏障的化学反应可能具有有趣的动态行为,这些行为与具有实质性屏障或完全由捕获主导的化学反应不同。 Si + + H 2 O 反应是一个典型的例子,甚至涉及反应路径上的两个浸没鞍点:一个用于 H 的直接解离(H 解离 SP),另一个用于 H 从 O 侧迁移到 Si -侧(H-迁移SP)。我们通过在精确的全维从头势能表面上采用准经典轨迹计算来研究这个过程的复杂性。通过仔细的轨迹分析,发现一个有趣的非本征反应配位机制在产生SiOH +和H方面发挥着重要作用。这条新途径的特点是H原子不会沿着最小能量路径形成HSiOH +配合物,而是直接解离通过H-迁移SP后进入产品。此外,基于人工缩放的势能面(PES),还探讨了势垒高度对反应的影响。这项工作为 Si + + H 2 O 反应动力学提供了新的见解,并丰富了我们对水下势垒反应的理解。
更新日期:2024-03-18
中文翻译:

浸没势垒双分子反应的非 IRC 机理:以 Si+ + H2O 反应为例
水下屏障的化学反应可能具有有趣的动态行为,这些行为与具有实质性屏障或完全由捕获主导的化学反应不同。 Si + + H 2 O 反应是一个典型的例子,甚至涉及反应路径上的两个浸没鞍点:一个用于 H 的直接解离(H 解离 SP),另一个用于 H 从 O 侧迁移到 Si -侧(H-迁移SP)。我们通过在精确的全维从头势能表面上采用准经典轨迹计算来研究这个过程的复杂性。通过仔细的轨迹分析,发现一个有趣的非本征反应配位机制在产生SiOH +和H方面发挥着重要作用。这条新途径的特点是H原子不会沿着最小能量路径形成HSiOH +配合物,而是直接解离通过H-迁移SP后进入产品。此外,基于人工缩放的势能面(PES),还探讨了势垒高度对反应的影响。这项工作为 Si + + H 2 O 反应动力学提供了新的见解,并丰富了我们对水下势垒反应的理解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号