当前位置:
X-MOL 学术
›
Pestic. Biochem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding properties of chemosensory protein 4 in Riptortus pedestris to aggregation pheromones
Pesticide Biochemistry and Physiology ( IF 4.7 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.pestbp.2024.105874 Jin-Bu Li , Qiang Liu , Sai Ma , Yue-Ying Wang , Xing-Zhou Liu , Chao-Wei Wang , Da-Jiang Wang , Zhuang-Zhuang Hu , Jia-Wen Gan , Xiu-Yun Zhu , Bao-Ping Li , Mao-Zhu Yin , Ya-Nan Zhang
Pesticide Biochemistry and Physiology ( IF 4.7 ) Pub Date : 2024-03-18 , DOI: 10.1016/j.pestbp.2024.105874 Jin-Bu Li , Qiang Liu , Sai Ma , Yue-Ying Wang , Xing-Zhou Liu , Chao-Wei Wang , Da-Jiang Wang , Zhuang-Zhuang Hu , Jia-Wen Gan , Xiu-Yun Zhu , Bao-Ping Li , Mao-Zhu Yin , Ya-Nan Zhang
|
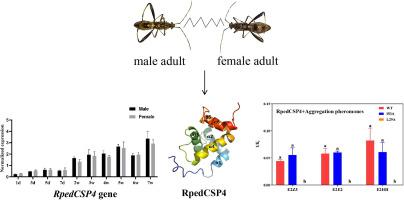
In insects, chemosensory proteins (CSPs) play an important role in the perception of the external environment and have been widely used for protein-binding characterization. has received increased attention as a potential cause of soybean staygreen syndrome in recent years. In this study, we found that expression in the antennae of adult increased with age, with no significant difference in expression level observed between males and females, as determined through quantitative real-time polymerase chain reaction (qRT-PCR). Subsequently, we investigated the ability of RpedCSP4 to bind various ligands (five aggregated pheromone components and 13 soybean volatiles) using a prokaryotic expression system and fluorescence competitive binding assays. We found that RpedCSP4 binds to three aggregated pheromone components of namely, ((E)-2-hexenyl (Z)-3-hexenoate (E2Z3), (E)-2-hexenyl (E)-2-hexenoate (E2E2), and (E)-2-hexenyl hexenoate (E2HH)), and that its binding capacities are most stable under acidic condition. Finally, the structure and protein-ligand interactions of RpedCSP4 were further analyzed via homology modeling, molecular docking, and targeted mutagenesis experiments. The L29A mutant exhibited a loss of binding ability to these three aggregated pheromone components. Our results show that the olfactory function of RpedCSP4 provides new insights into the binding mechanism of RpedCSPs to aggregation pheromones and contributes to discover new target candidates that will provide a theoretical basis for future population control of .
中文翻译:

Riptortus pedestris 化学感应蛋白 4 与聚集信息素的结合特性
在昆虫中,化学感应蛋白(CSP)在感知外部环境中发挥着重要作用,并已广泛用于蛋白质结合表征。近年来作为大豆常绿综合症的潜在原因受到越来越多的关注。在这项研究中,我们发现成虫触角中的表达随着年龄的增长而增加,通过定量实时聚合酶链反应(qRT-PCR)测定,男性和女性之间的表达水平没有显着差异。随后,我们使用原核表达系统和荧光竞争性结合测定研究了 RpedCSP4 结合各种配体(五种聚集信息素成分和 13 种大豆挥发物)的能力。我们发现 RpedCSP4 与三种聚集的信息素成分结合,即 ((E)-2-己烯基 (Z)-3-己烯酸酯 (E2Z3)、(E)-2-己烯基 (E)-2-己烯酸酯 (E2E2)、和(E)-2-己烯基己烯酸酯(E2HH)),其结合能力在酸性条件下最稳定。最后,通过同源建模、分子对接和靶向诱变实验进一步分析了RpedCSP4的结构和蛋白质-配体相互作用。 L29A突变体表现出对这三种聚集信息素成分的结合能力的丧失。我们的研究结果表明,RpedCSP4的嗅觉功能为RpedCSP与聚集信息素的结合机制提供了新的见解,并有助于发现新的候选目标,为未来的种群控制提供理论基础。
更新日期:2024-03-18
中文翻译:

Riptortus pedestris 化学感应蛋白 4 与聚集信息素的结合特性
在昆虫中,化学感应蛋白(CSP)在感知外部环境中发挥着重要作用,并已广泛用于蛋白质结合表征。近年来作为大豆常绿综合症的潜在原因受到越来越多的关注。在这项研究中,我们发现成虫触角中的表达随着年龄的增长而增加,通过定量实时聚合酶链反应(qRT-PCR)测定,男性和女性之间的表达水平没有显着差异。随后,我们使用原核表达系统和荧光竞争性结合测定研究了 RpedCSP4 结合各种配体(五种聚集信息素成分和 13 种大豆挥发物)的能力。我们发现 RpedCSP4 与三种聚集的信息素成分结合,即 ((E)-2-己烯基 (Z)-3-己烯酸酯 (E2Z3)、(E)-2-己烯基 (E)-2-己烯酸酯 (E2E2)、和(E)-2-己烯基己烯酸酯(E2HH)),其结合能力在酸性条件下最稳定。最后,通过同源建模、分子对接和靶向诱变实验进一步分析了RpedCSP4的结构和蛋白质-配体相互作用。 L29A突变体表现出对这三种聚集信息素成分的结合能力的丧失。我们的研究结果表明,RpedCSP4的嗅觉功能为RpedCSP与聚集信息素的结合机制提供了新的见解,并有助于发现新的候选目标,为未来的种群控制提供理论基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号