当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthetic Studies toward Pseudolaric Acids: Radical Cyclization to Form Bridged Scaffold
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2024-03-19 , DOI: 10.1002/cjoc.202400085 Yujie Niu 1 , Minggui Lin 1 , Hao Cui 1 , Yanji Huang 1 , Yang Shen 1 , Yandong Zhang 1
Chinese Journal of Chemistry ( IF 5.4 ) Pub Date : 2024-03-19 , DOI: 10.1002/cjoc.202400085 Yujie Niu 1 , Minggui Lin 1 , Hao Cui 1 , Yanji Huang 1 , Yang Shen 1 , Yandong Zhang 1
Affiliation
|
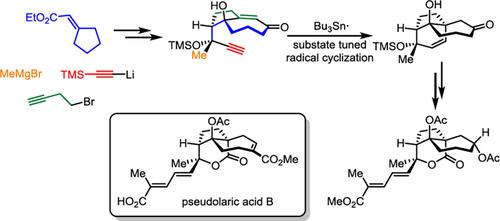
Comprehensive SummaryPseudolaric acids are a family of diterpenoid natural products that exhibit a broad spectrum of biological activities. The main structural feature of their framework is a trans ‐fused perhydroazulene bearing a bridged lactone positioned at the junction of the rings. Herein, we have developed a radical cyclization strategy that allows flexible tuning of the cyclization process through diverse silicon substitutions on the substrates. This strategy can assist in constructing a series of skeletons with structural resemblance to pseudolaric acids and expedites the construction of the bridged lactone. Finally, it facilitates the synthesis of the entire skeletal structure of the pseudolaric acid family of natural products, excluding the B‐ring functionalization.
中文翻译:

拟二酸的合成研究:自由基环化形成桥支架
综合摘要假油酸是一类二萜类天然产物,具有广泛的生物活性。他们的框架的主要结构特征是反式 ‐稠合全氢甘菊环,在环的连接处带有桥连内酯。在这里,我们开发了一种自由基环化策略,可以通过基板上的不同硅取代来灵活调整环化过程。该策略可以帮助构建一系列与假月桂酸结构相似的骨架,并加速桥连内酯的构建。最后,它促进了伪月桂酸家族天然产物的整个骨架结构的合成,不包括B环官能化。
更新日期:2024-03-19
中文翻译:

拟二酸的合成研究:自由基环化形成桥支架
综合摘要假油酸是一类二萜类天然产物,具有广泛的生物活性。他们的框架的主要结构特征是



























 京公网安备 11010802027423号
京公网安备 11010802027423号