当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic Behavior of the (Methylcyclopentane + Ethyl Acetate) Binary Liquid Mixture: Density at Several Temperatures and Vapor–Liquid Equilibrium at 25, 50, and 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.jced.4c00013 Kaoutar Berkalou 1, 2 , Vincent Caqueret 1, 2 , Gabriela Zanghelini 3 , Stéphane Vitu 1, 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2024-03-19 , DOI: 10.1021/acs.jced.4c00013 Kaoutar Berkalou 1, 2 , Vincent Caqueret 1, 2 , Gabriela Zanghelini 3 , Stéphane Vitu 1, 2
Affiliation
|
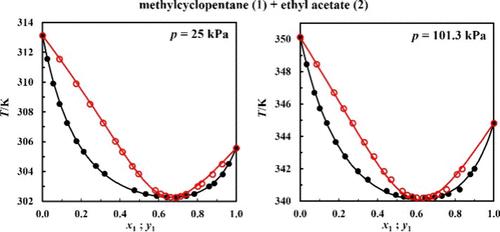
The density and isobaric phase equilibria of the binary system methylcyclopentane + ethyl acetate were investigated for the first time in this work. The density of the system was measured at 101 kPa from 288.15 to 308.15 K for the entire composition range thanks to a vibrating tube densimeter. Positive excess molar volumes were observed and correlated by using a Redlich–Kister equation. New isobaric vapor–liquid equilibrium data for the system methylcyclopentane + ethyl acetate were measured by means of a recirculation ebulliometer. The phase equilibrium was measured at 25, 50, and 101.3 kPa. Equilibrium compositions were determined indirectly from density measurements of the liquid and condensed vapor phases. Some additional density and vapor–liquid equilibrium data measurements for the binary system ethyl acetate + methylcyclohexane were performed for comparison with previously published results and validation of the experimental protocol. The methylcyclopentane + ethyl acetate binary mixture displays pronounced positive deviations from ideality and a positive azeotrope. Three thermodynamic consistency tests were employed to validate the produced data set. The reported data were successfully correlated by the NRTL model, and the modified UNIFAC predictive model was also used. UNIFAC provides a very good description of the phase behavior at 101.3 kPa, whereas predictions are less accurate at low pressures (25 and 50 kPa).
中文翻译:

(甲基环戊烷 + 乙酸乙酯)二元液体混合物的热力学行为:多个温度下的密度以及 25、50 和 101.3 kPa 下的汽液平衡
本工作首次研究了甲基环戊烷+乙酸乙酯二元体系的密度和等量异位相平衡。通过振动管密度计,在 288.15 至 308.15 K 的整个成分范围内,在 101 kPa 下测量了系统的密度。使用 Redlich-Kister 方程观察并关联正过量摩尔体积。甲基环戊烷+乙酸乙酯系统的新等压汽液平衡数据通过再循环沸点计测量。在 25、50 和 101.3 kPa 下测量相平衡。通过液相和冷凝气相的密度测量间接确定平衡组成。对二元系统乙酸乙酯+甲基环己烷进行了一些额外的密度和气液平衡数据测量,以与之前发布的结果进行比较并验证实验方案。甲基环戊烷+乙酸乙酯二元混合物表现出与理想状态的明显正偏差和正共沸物。采用三个热力学一致性测试来验证生成的数据集。报告的数据通过 NRTL 模型成功关联,并且还使用了修改后的 UNIFAC 预测模型。 UNIFAC 对 101.3 kPa 下的相行为提供了非常好的描述,而在低压(25 和 50 kPa)下的预测不太准确。
更新日期:2024-03-19
中文翻译:

(甲基环戊烷 + 乙酸乙酯)二元液体混合物的热力学行为:多个温度下的密度以及 25、50 和 101.3 kPa 下的汽液平衡
本工作首次研究了甲基环戊烷+乙酸乙酯二元体系的密度和等量异位相平衡。通过振动管密度计,在 288.15 至 308.15 K 的整个成分范围内,在 101 kPa 下测量了系统的密度。使用 Redlich-Kister 方程观察并关联正过量摩尔体积。甲基环戊烷+乙酸乙酯系统的新等压汽液平衡数据通过再循环沸点计测量。在 25、50 和 101.3 kPa 下测量相平衡。通过液相和冷凝气相的密度测量间接确定平衡组成。对二元系统乙酸乙酯+甲基环己烷进行了一些额外的密度和气液平衡数据测量,以与之前发布的结果进行比较并验证实验方案。甲基环戊烷+乙酸乙酯二元混合物表现出与理想状态的明显正偏差和正共沸物。采用三个热力学一致性测试来验证生成的数据集。报告的数据通过 NRTL 模型成功关联,并且还使用了修改后的 UNIFAC 预测模型。 UNIFAC 对 101.3 kPa 下的相行为提供了非常好的描述,而在低压(25 和 50 kPa)下的预测不太准确。



























 京公网安备 11010802027423号
京公网安备 11010802027423号