当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single-Nucleobase-Resolved Nanoruler Determines the Surface Energy Transfer Radius on the Living Cell Membrane
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.analchem.4c00128 Wenwen Huang 1 , Yu Zhang 1 , Xingru Fang 1 , Qi Li 1 , Honglin Liu 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.analchem.4c00128 Wenwen Huang 1 , Yu Zhang 1 , Xingru Fang 1 , Qi Li 1 , Honglin Liu 1
Affiliation
|
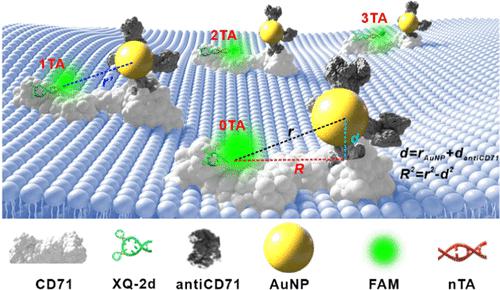
Investigations about surface energy transfer radius (r0) are limited to the aqueous solution system, and it is quite limited on experimental values of r0 between dyes and the corresponding gold particle (AuNP) sizes, especially for living cell systems. Hence, the selection of suitable AuNP-dye pairs is restricted when designing nanometal surface energy transfer (NSET) strategies in analytical sciences. Here, we developed a single-nucleobase-resolved NSET strategy to in situ measure the r0 value between a specific dye and different-sized AuNPs on the living cell membrane. Using the aptamer-dye complex (XQ-2d-nTA-FAM) and antiCD71 antibody-coupled AuNP conjugate (Au@antiCD71) as two working elements to bind two different sites on CD71 receptors on living cell membranes, we modified the nTA spacer between FAM and the terminal of aptamer to change the distance (r) from FAM to AuNP center and further adjusted the quenching efficiency (Φ) between them. Different r0 values of various AuNP-FAM pairs in living cells are determined by this in situ detection strategy. Based on this single-nucleobase-resolved NSET strategy, we established a simple and efficient universal method for measuring r0 in the living cell system, which greatly expanded the selection range of AuNP-dye pairs during the construction of the NSET model at the nanoscale.
中文翻译:

单核碱基解析的纳米尺确定活细胞膜上的表面能量转移半径
关于表面能转移半径(r 0)的研究仅限于水溶液体系,并且染料之间的r 0实验值和相应的金颗粒(AuNP)尺寸相当有限,特别是对于活细胞体系。因此,在分析科学中设计纳米金属表面能量转移(NSET)策略时,合适的金纳米粒子染料对的选择受到限制。在这里,我们开发了一种单核碱基解析的 NSET 策略,用于原位测量活细胞膜上特定染料和不同大小的 AuNP 之间的r 0值。使用适体-染料复合物(XQ-2d- n TA-FAM)和抗CD71抗体偶联的AuNP缀合物(Au@antiCD71)作为两个工作元件来结合活细胞膜上CD71受体上的两个不同位点,我们修饰了n TA FAM和适配体末端之间的间隔物改变FAM到AuNP中心的距离( r ),并进一步调节它们之间的猝灭效率(Φ)。通过这种原位检测策略确定活细胞中各种AuNP-FAM对的不同r 0值。基于这种单核碱基解析的NSET策略,我们建立了一种简单高效的活细胞系统中测量r 0 的通用方法,极大地扩展了纳米级NSET模型构建过程中AuNP-染料对的选择范围。
更新日期:2024-03-20
中文翻译:

单核碱基解析的纳米尺确定活细胞膜上的表面能量转移半径
关于表面能转移半径(r 0)的研究仅限于水溶液体系,并且染料之间的r 0实验值和相应的金颗粒(AuNP)尺寸相当有限,特别是对于活细胞体系。因此,在分析科学中设计纳米金属表面能量转移(NSET)策略时,合适的金纳米粒子染料对的选择受到限制。在这里,我们开发了一种单核碱基解析的 NSET 策略,用于原位测量活细胞膜上特定染料和不同大小的 AuNP 之间的r 0值。使用适体-染料复合物(XQ-2d- n TA-FAM)和抗CD71抗体偶联的AuNP缀合物(Au@antiCD71)作为两个工作元件来结合活细胞膜上CD71受体上的两个不同位点,我们修饰了n TA FAM和适配体末端之间的间隔物改变FAM到AuNP中心的距离( r ),并进一步调节它们之间的猝灭效率(Φ)。通过这种原位检测策略确定活细胞中各种AuNP-FAM对的不同r 0值。基于这种单核碱基解析的NSET策略,我们建立了一种简单高效的活细胞系统中测量r 0 的通用方法,极大地扩展了纳米级NSET模型构建过程中AuNP-染料对的选择范围。



























 京公网安备 11010802027423号
京公网安备 11010802027423号