当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chiral Recognition of Chiral (Hetero)Cyclic Derivatives Probed by Tetraaza Macrocyclic Chiral Solvating Agents via 1H NMR Spectroscopy
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.analchem.3c05395 Yu Wang 1 , Hongmei Zhao 2 , Chunxia Yang 1 , Lixia Fang 1 , Li Zheng 1 , Hehua Lv 1 , Pericles Stavropoulos 3 , Lin Ai 1 , Jiaxin Zhang 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2024-03-20 , DOI: 10.1021/acs.analchem.3c05395 Yu Wang 1 , Hongmei Zhao 2 , Chunxia Yang 1 , Lixia Fang 1 , Li Zheng 1 , Hehua Lv 1 , Pericles Stavropoulos 3 , Lin Ai 1 , Jiaxin Zhang 1
Affiliation
|
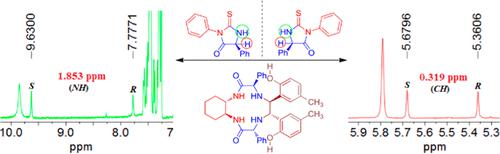
In the field of chiral recognition, chiral cyclic organic compounds, especially heterocyclic organic compounds, have attracted little attention and have been rarely studied as chiral substrates by means of 1H NMR spectroscopy. In this paper, enantiomers of thiohydantoin derivatives, representing typical five-membered N,N-heterocycles, have been synthesized and utilized for assignment of absolute configuration and analysis of enantiomeric excess. All enantiomers have been successfully differentiated with the assistance of novel tetraaza macrocyclic chiral solvating agents (TAMCSAs) by 1H NMR spectroscopy. Surprisingly, unprecedented nonequivalent chemical shift values (up to 2.052 ppm) of the NH proton of substrates have been observed, a new milestone in the evaluation of enantiomers. To better understand the intermolecular interactions between host and guest, Job plots and theoretical calculations of (S)-G1 and (R)-G1 with TAMCSA 1a were investigated and revealed significant geometric differentiation between the diastereomers. In order to evaluate practical applications of the present systems in analyzing optical purity of chiral substrates, enantiomeric excesses of a typical substrate (G1) with different optical compositions in the presence of a representative TAMCSA (1a) can be accurately calculated based on the integration of the NH proton’s signal peaks. Importantly, this work provides a significant breakthrough in exploring and developing the chiral recognition of chiral heterocyclic organic compounds by 1H NMR spectroscopy.
中文翻译:

四氮杂大环手性溶剂化剂通过 1H NMR 光谱探测手性(杂)环衍生物的手性识别
在手性识别领域,手性环状有机化合物,特别是杂环有机化合物很少引起人们的关注,并且很少通过1 H NMR 光谱研究作为手性底物。本文合成了代表典型五元N , N-杂环的乙内酰硫脲衍生物的对映体,并将其用于绝对构型的分配和对映体过量的分析。在新型四氮杂大环手性溶剂化剂 (TAMCSA) 的帮助下,所有对映体均已通过1 H NMR 光谱成功区分。令人惊讶的是,我们观察到了底物的 NH 质子前所未有的非当量化学位移值(高达 2.052 ppm),这是对映体评估中的一个新里程碑。为了更好地理解主体和客体之间的分子间相互作用,研究了 ( S )-G 1和 ( R )-G 1与 TAMCSA 1a的工作图和理论计算,并揭示了非对映异构体之间显着的几何差异。为了评估本系统在分析手性底物光学纯度方面的实际应用,可以基于积分准确计算在代表性 TAMCSA ( 1a )存在下具有不同光学组成的典型底物 (G 1 ) 的对映体过量。 NH 质子的信号峰。重要的是,这项工作为探索和发展1 H NMR光谱手性杂环有机化合物的手性识别提供了重大突破。
更新日期:2024-03-20
中文翻译:

四氮杂大环手性溶剂化剂通过 1H NMR 光谱探测手性(杂)环衍生物的手性识别
在手性识别领域,手性环状有机化合物,特别是杂环有机化合物很少引起人们的关注,并且很少通过1 H NMR 光谱研究作为手性底物。本文合成了代表典型五元N , N-杂环的乙内酰硫脲衍生物的对映体,并将其用于绝对构型的分配和对映体过量的分析。在新型四氮杂大环手性溶剂化剂 (TAMCSA) 的帮助下,所有对映体均已通过1 H NMR 光谱成功区分。令人惊讶的是,我们观察到了底物的 NH 质子前所未有的非当量化学位移值(高达 2.052 ppm),这是对映体评估中的一个新里程碑。为了更好地理解主体和客体之间的分子间相互作用,研究了 ( S )-G 1和 ( R )-G 1与 TAMCSA 1a的工作图和理论计算,并揭示了非对映异构体之间显着的几何差异。为了评估本系统在分析手性底物光学纯度方面的实际应用,可以基于积分准确计算在代表性 TAMCSA ( 1a )存在下具有不同光学组成的典型底物 (G 1 ) 的对映体过量。 NH 质子的信号峰。重要的是,这项工作为探索和发展1 H NMR光谱手性杂环有机化合物的手性识别提供了重大突破。



























 京公网安备 11010802027423号
京公网安备 11010802027423号