当前位置:
X-MOL 学术
›
Atherosclerosis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Applicability and impact of the COMPASS trial in a Canadian population of patients with atherosclerotic disease
Atherosclerosis ( IF 5.3 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.atherosclerosis.2024.117486 Robert C. Welsh , Pishoy Gouda , Doug Dover , Kevin R. Bainey , Finlay A. McAlister , Padma Kaul
Atherosclerosis ( IF 5.3 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.atherosclerosis.2024.117486 Robert C. Welsh , Pishoy Gouda , Doug Dover , Kevin R. Bainey , Finlay A. McAlister , Padma Kaul
|
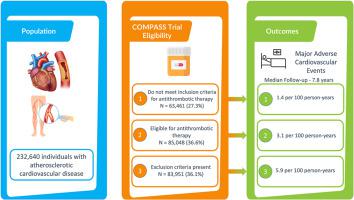
In the COMPASS trial, low-dose rivaroxaban with aspirin improved cardiovascular outcomes in patients with atherosclerotic cardiovascular disease (ASCVD). We aimed to assess the potential clinical implications of this therapy in a generalizable population. A retrospective cohort of adults with ASVCD was formed using healthcare administrative databases in Alberta, Canada (population 4.4 million). Patients with a new diagnosis between 2008 and 2019 formed the epidemiological cohort (n = 224,600) and those with long-term follow-up (>5 years) formed the outcomes cohort (n = 232,460). The primary outcome of major adverse cardiovascular events (MACE) was assessed and categorized based on the COMPASS trial eligibility. In the outcomes cohort, 77% had only coronary artery disease, 15% had only peripheral artery disease, and 8% had both. Of those, 37% met the COMPASS trial eligibility criteria, 36% met exclusion criteria and 27% did not meet inclusion criteria. Over a median of 7.8 years, the COMPASS exclusion group demonstrated the highest rate of MACE (5.9 per 100 person-years), following by the eligible group and the group that did not meet COMPASS inclusion criteria (3.1 and 1.4 per 100 person-years respectively). The expected net clinical benefit of antithrombotic therapy in the eligible group was 5.6 fewer events per 1000 person-years. In a real-world population of 4.4 million adults, there are roughly 20,000 new cases of ASVCD diagnosed yearly, with ∼40% being eligible for the addition of low-dose rivaroxaban therapy to antiplatelet therapy. The theoretical implementation of dual antithrombotic treatment in this population could result in a substantial reduction in cardiovascular morbidity and mortality.
中文翻译:

COMPASS 试验在加拿大动脉粥样硬化疾病患者群体中的适用性和影响
在 COMPASS 试验中,低剂量利伐沙班联合阿司匹林改善了动脉粥样硬化性心血管疾病 (ASCVD) 患者的心血管结局。我们的目的是评估这种疗法在一般人群中的潜在临床意义。使用加拿大艾伯塔省的医疗保健管理数据库(人口 440 万)形成了 ASVCD 成人回顾性队列。 2008 年至 2019 年间新诊断的患者构成流行病学队列(n = 224,600),长期随访(> 5 年)的患者构成结果队列(n = 232,460)。主要不良心血管事件 (MACE) 的主要结局根据 COMPASS 试验资格进行评估和分类。在结果队列中,77% 的人仅患有冠状动脉疾病,15% 的人仅患有外周动脉疾病,8% 的人两者都有。其中,37% 符合 COMPASS 试验资格标准,36% 符合排除标准,27% 不符合纳入标准。在中位数 7.8 年的时间里,COMPASS 排除组的 MACE 发生率最高(每 100 人年 5.9 例),其次是符合条件的组和不符合 COMPASS 纳入标准的组(每 100 人年 3.1 例和 1.4 例)分别)。在合格组中,抗血栓治疗的预期临床净效益为每 1000 人年减少 5.6 次事件。在 440 万成年人的现实世界中,每年大约诊断出 20,000 例 ASVCD 新病例,其中约 40% 的患者有资格在抗血小板治疗的基础上加用低剂量利伐沙班治疗。理论上,在该人群中实施双重抗血栓治疗可能会大幅降低心血管发病率和死亡率。
更新日期:2024-03-16
中文翻译:

COMPASS 试验在加拿大动脉粥样硬化疾病患者群体中的适用性和影响
在 COMPASS 试验中,低剂量利伐沙班联合阿司匹林改善了动脉粥样硬化性心血管疾病 (ASCVD) 患者的心血管结局。我们的目的是评估这种疗法在一般人群中的潜在临床意义。使用加拿大艾伯塔省的医疗保健管理数据库(人口 440 万)形成了 ASVCD 成人回顾性队列。 2008 年至 2019 年间新诊断的患者构成流行病学队列(n = 224,600),长期随访(> 5 年)的患者构成结果队列(n = 232,460)。主要不良心血管事件 (MACE) 的主要结局根据 COMPASS 试验资格进行评估和分类。在结果队列中,77% 的人仅患有冠状动脉疾病,15% 的人仅患有外周动脉疾病,8% 的人两者都有。其中,37% 符合 COMPASS 试验资格标准,36% 符合排除标准,27% 不符合纳入标准。在中位数 7.8 年的时间里,COMPASS 排除组的 MACE 发生率最高(每 100 人年 5.9 例),其次是符合条件的组和不符合 COMPASS 纳入标准的组(每 100 人年 3.1 例和 1.4 例)分别)。在合格组中,抗血栓治疗的预期临床净效益为每 1000 人年减少 5.6 次事件。在 440 万成年人的现实世界中,每年大约诊断出 20,000 例 ASVCD 新病例,其中约 40% 的患者有资格在抗血小板治疗的基础上加用低剂量利伐沙班治疗。理论上,在该人群中实施双重抗血栓治疗可能会大幅降低心血管发病率和死亡率。



























 京公网安备 11010802027423号
京公网安备 11010802027423号