当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, biological evaluation and molecular docking study of pyrimidine linked thiazolidinedione derivatives as potential antimicrobial and antitubercular agents
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-03-15 , DOI: 10.1016/j.bmcl.2024.129707 M.S. Raghu , C.B. Pradeep Kumar , K Yogesh Kumar , M.K. Prashanth , Fahd Alharethy , Byong-Hun Jeon
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2024-03-15 , DOI: 10.1016/j.bmcl.2024.129707 M.S. Raghu , C.B. Pradeep Kumar , K Yogesh Kumar , M.K. Prashanth , Fahd Alharethy , Byong-Hun Jeon
|
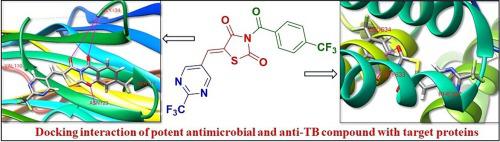
The design and development of novel antimicrobial agents are highly desired to combat the emergence of medication resistance against microorganisms that cause infections. A series of new pyrimidine-linked thiazolidinedione derivatives () were synthesized, characterized, and their antimicrobial properties assessed in the current investigation. Here, novel pyrimidine-linked thiazolidinedione compounds were designed using the molecular hybridization approach. Elemental and spectral techniques were used to determine the structures of the synthesized hybrids. The majority of compounds showed encouraging antibacterial properties. Among the active compounds, , , and showed 1.85, 1.15, and 1.38 times the activity of streptomycin against , respectively, with MIC values of 6.4, 10.3, and 8.6 µM. With MIC values of 10.8, 21.9, and 15.4 µM, respectively, the compounds , , and showed 2.14, 1.05, and 1.50 times the activity of linezolid against the methicillin-resistant (MRSA) strain. Furthermore, when compared to the reference medications, compounds , , and demonstrated broad-range antimicrobial efficacy against all tested strains of bacteria and fungus. Out of all the compounds that were investigated, compounds , , and showed noteworthy anti-tubercular activity. is the most effective, 1.59 times more effective than reference drug isoniazid. To anticipate the binding manner, the synthesized potent compounds were subjected to molecular docking into the active binding site of MRSA and the mycobacterial membrane protein large 3 (MmpL3) protein. The compounds , , and may eventually serve as lead compounds in the search for antimicrobial and anti-TB therapeutic agents.
中文翻译:

嘧啶连接的噻唑烷二酮衍生物作为潜在抗菌和抗结核药物的合成、生物学评价和分子对接研究
迫切需要设计和开发新型抗菌剂来对抗引起感染的微生物的耐药性的出现。在当前的研究中,合成、表征了一系列新的嘧啶连接的噻唑烷二酮衍生物(),并评估了它们的抗菌特性。在这里,使用分子杂交方法设计了新型嘧啶连接的噻唑烷二酮化合物。使用元素和光谱技术来确定合成杂化物的结构。大多数化合物显示出令人鼓舞的抗菌特性。在活性化合物中, 、 、 和 对 的活性分别是链霉素的 1.85、1.15 和 1.38 倍,MIC 值为 6.4、10.3 和 8.6 µM。化合物 、 、 和 的 MIC 值分别为 10.8、21.9 和 15.4 µM,其对抗甲氧西林 (MRSA) 菌株的活性是利奈唑胺的 2.14、1.05 和 1.50 倍。此外,与参考药物相比,化合物、、和对所有测试的细菌和真菌菌株表现出广泛的抗菌功效。在所有被研究的化合物中,化合物 、 、 和 显示出显着的抗结核活性。最有效,比参考药异烟肼有效1.59倍。为了预测结合方式,将合成的有效化合物进行分子对接至 MRSA 和分枝杆菌膜蛋白大 3 (MmpL3) 蛋白的活性结合位点。这些化合物、、和最终可能成为寻找抗菌和抗结核治疗药物的先导化合物。
更新日期:2024-03-15
中文翻译:

嘧啶连接的噻唑烷二酮衍生物作为潜在抗菌和抗结核药物的合成、生物学评价和分子对接研究
迫切需要设计和开发新型抗菌剂来对抗引起感染的微生物的耐药性的出现。在当前的研究中,合成、表征了一系列新的嘧啶连接的噻唑烷二酮衍生物(),并评估了它们的抗菌特性。在这里,使用分子杂交方法设计了新型嘧啶连接的噻唑烷二酮化合物。使用元素和光谱技术来确定合成杂化物的结构。大多数化合物显示出令人鼓舞的抗菌特性。在活性化合物中, 、 、 和 对 的活性分别是链霉素的 1.85、1.15 和 1.38 倍,MIC 值为 6.4、10.3 和 8.6 µM。化合物 、 、 和 的 MIC 值分别为 10.8、21.9 和 15.4 µM,其对抗甲氧西林 (MRSA) 菌株的活性是利奈唑胺的 2.14、1.05 和 1.50 倍。此外,与参考药物相比,化合物、、和对所有测试的细菌和真菌菌株表现出广泛的抗菌功效。在所有被研究的化合物中,化合物 、 、 和 显示出显着的抗结核活性。最有效,比参考药异烟肼有效1.59倍。为了预测结合方式,将合成的有效化合物进行分子对接至 MRSA 和分枝杆菌膜蛋白大 3 (MmpL3) 蛋白的活性结合位点。这些化合物、、和最终可能成为寻找抗菌和抗结核治疗药物的先导化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号