当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient assembly and anti-tumor evaluation of novel polycyclic [1,2-a]-fused indoles
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.bioorg.2024.107289 Hui Guo , Yuqi Tian , Xing Wu , Liang Tu , Jikai Liu , Yongsheng Zheng , Rong Huang
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2024-03-16 , DOI: 10.1016/j.bioorg.2024.107289 Hui Guo , Yuqi Tian , Xing Wu , Liang Tu , Jikai Liu , Yongsheng Zheng , Rong Huang
|
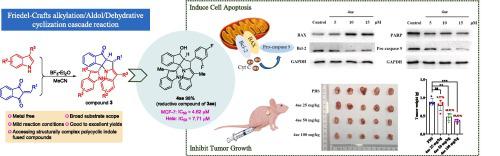
Structurally diverse cyclopenta[4,5]pyrrolo[1,2-]indoles heterocycles were smoothly constructed in good to excellent yields (up to 99 %) with excellent diastereoselectivities (>19:1 dr) through a novel and facile strategy based on BF-catalyzed Friedel-Crafts alkylation/Aldol/Dehydrative cyclization cascade reaction. The anti-proliferative activity of these newly synthesized polycyclic indoles was screened, and all the functionalized reductive derivatives exhibited favorable anti-tumor activity. Notably, compound 4ae displayed the remarkable inhibitory activity against MCF-7 and HeLa cells with IC values of 4.62 μM and 7.71 μM, respectively. Mechanistically, the representative compound 4ae could effectively induce apoptosis of MCF-7 cells in crediting to up-regulate the relative expression of apoptotic protein BAX/Bcl-2, subsequently activate Pro-caspase 9 and cleave PARP, simultaneously block the cell cycle through down- and up-regulate the expression of cyclin B1 and p53, respectively. Moreover, compound 4ae also exhibited promising antineoplastic efficacy in subcutaneous MCF-7 xenograft mice which manifest significant shrunken tumors conspicuous nuclear apoptotic signal and minimal systemic toxicity. This strategy not only established a novel and efficient method for the assembly of structurally complex indole heterocycles, but also provided a series of compounds possessing attractive anti-cancer activity, which holds immense potential for future biomedical applications.
中文翻译:

新型多环[1,2-a]-稠合吲哚的高效组装及抗肿瘤评价
通过基于 BF 的新颖且简便的策略,可以顺利构建结构多样的环戊[4,5]吡咯并[1,2-]吲哚杂环,产率良好至优异(高达 99%),具有优异的非对映选择性(>19:1 dr) -催化Friedel-Crafts烷基化/羟醛/脱水环化级联反应。对这些新合成的多环吲哚的抗增殖活性进行了筛选,所有功能化的还原衍生物均表现出良好的抗肿瘤活性。值得注意的是,化合物 4ae 对 MCF-7 和 HeLa 细胞表现出显着的抑制活性,IC 值分别为 4.62 μM 和 7.71 μM。机理上,代表性化合物4ae可有效诱导MCF-7细胞凋亡,其原因是上调凋亡蛋白BAX/Bcl-2的相对表达,随后激活Pro-caspase 9并裂解PARP,同时下调细胞周期。 - 并分别上调细胞周期蛋白 B1 和 p53 的表达。此外,化合物4ae还在皮下MCF-7异种移植小鼠中表现出有希望的抗肿瘤功效,其表现出显着缩小的肿瘤、明显的核凋亡信号和最小的全身毒性。该策略不仅为结构复杂的吲哚杂环的组装建立了一种新颖有效的方法,而且提供了一系列具有有吸引力的抗癌活性的化合物,这在未来的生物医学应用中具有巨大的潜力。
更新日期:2024-03-16
中文翻译:

新型多环[1,2-a]-稠合吲哚的高效组装及抗肿瘤评价
通过基于 BF 的新颖且简便的策略,可以顺利构建结构多样的环戊[4,5]吡咯并[1,2-]吲哚杂环,产率良好至优异(高达 99%),具有优异的非对映选择性(>19:1 dr) -催化Friedel-Crafts烷基化/羟醛/脱水环化级联反应。对这些新合成的多环吲哚的抗增殖活性进行了筛选,所有功能化的还原衍生物均表现出良好的抗肿瘤活性。值得注意的是,化合物 4ae 对 MCF-7 和 HeLa 细胞表现出显着的抑制活性,IC 值分别为 4.62 μM 和 7.71 μM。机理上,代表性化合物4ae可有效诱导MCF-7细胞凋亡,其原因是上调凋亡蛋白BAX/Bcl-2的相对表达,随后激活Pro-caspase 9并裂解PARP,同时下调细胞周期。 - 并分别上调细胞周期蛋白 B1 和 p53 的表达。此外,化合物4ae还在皮下MCF-7异种移植小鼠中表现出有希望的抗肿瘤功效,其表现出显着缩小的肿瘤、明显的核凋亡信号和最小的全身毒性。该策略不仅为结构复杂的吲哚杂环的组装建立了一种新颖有效的方法,而且提供了一系列具有有吸引力的抗癌活性的化合物,这在未来的生物医学应用中具有巨大的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号